��Ŀ����
�±��зֱ���̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ��
| �¶ȡ� | 0 | 10 | 20 | 30 | 40 | 60 | 80 | |
| �ܽ��/g | Na2CO3 | 7.00 | 12.5 | 21.5 | 39.7 | 49.0 | 46.0 | 43.9 |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.3 | 38.4 | |
��̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ�ȣ���ش�
��1��10��ʱ��̼���Ʊ��Ȼ��Ƶ��ܽ��______��
��2������ͼ�����ݣ������̼��ԭ����______��
��3��80��ʱ����100gˮ���Ƴ�̼���Ƶı�����Һ�����������������ǣ���ȷ������0.1%��______�����ø���Һ��������ʯ��ˮ��Ӧ���ɵ��������ƣ���ȷ��������______g��
�⣺
��1��10��ʱ̼���Ƶ��ܽ����12.5g���Ȼ�����35.8g�����ԿɱȽ��ܽ�ȵĴ�С��
��2����̼���Ƶ��ܽ�����¶Ƚ��Ͷ����Լ�С�������¶Ƚϵͣ�������Һ�нᾧ����������
��3��100gˮ����80��ʱ�ܽ��̼���Ƶ�������43.9g����Һ���ñ��ͣ���������������= ��100%=30.5%��
��100%=30.5%��
����43.9g̼������ʯ��ˮ��Ӧʱ�������������Ƶ�������x
Na2CO3+Ca��OH��2=2NaOH+CaCO3��
106 2��40
43.9g X
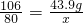
x=33g
�𣺿ɵ��������Ƶ�������33g��
�ʴ�Ϊ��
��1����
��2���������µͣ�������ܽ�����¶ȱ仯�����Լ�С�������ں�ˮ���γɱ�����Һ�����������壮
��3��30.5%��33��
��������1������10��ʱ�����ʵ��ܽ�ȼ��ɣ�
��2�����ô�����ܽ�����¶ȱ仯����������
��3������80��ʱ̼���Ƶ��ܽ�Ƚ�������ݻ�ѧ����ʽ�ļ�����֪��Ӧ���֪�����
�����������ѶȲ�����Ҫ�����˸��ݹ�����ܽ��ͼ�������ص����⣬�Ӷ�����ѧ����֪ʶ�������Ӧ�ã�
��1��10��ʱ̼���Ƶ��ܽ����12.5g���Ȼ�����35.8g�����ԿɱȽ��ܽ�ȵĴ�С��
��2����̼���Ƶ��ܽ�����¶Ƚ��Ͷ����Լ�С�������¶Ƚϵͣ�������Һ�нᾧ����������
��3��100gˮ����80��ʱ�ܽ��̼���Ƶ�������43.9g����Һ���ñ��ͣ���������������=
 ��100%=30.5%��
��100%=30.5%������43.9g̼������ʯ��ˮ��Ӧʱ�������������Ƶ�������x
Na2CO3+Ca��OH��2=2NaOH+CaCO3��
106 2��40
43.9g X
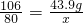
x=33g
�𣺿ɵ��������Ƶ�������33g��
�ʴ�Ϊ��
��1����
��2���������µͣ�������ܽ�����¶ȱ仯�����Լ�С�������ں�ˮ���γɱ�����Һ�����������壮
��3��30.5%��33��
��������1������10��ʱ�����ʵ��ܽ�ȼ��ɣ�
��2�����ô�����ܽ�����¶ȱ仯����������
��3������80��ʱ̼���Ƶ��ܽ�Ƚ�������ݻ�ѧ����ʽ�ļ�����֪��Ӧ���֪�����
�����������ѶȲ�����Ҫ�����˸��ݹ�����ܽ��ͼ�������ص����⣬�Ӷ�����ѧ����֪ʶ�������Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
�±��зֱ���̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ��
�ҹ��������μ�����������д����Ĵ�����Ȼ��ƣ������ũ�����̼����ɹ�Σ�
��̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ�ȣ���ش�
��1��10��ʱ��̼���Ʊ��Ȼ��Ƶ��ܽ�� ��
��2������ͼ�����ݣ������̼��ԭ���� ��
��3��80��ʱ����100gˮ���Ƴ�̼���Ƶı�����Һ�����������������ǣ���ȷ������0.1%�� �����ø���Һ��������ʯ��ˮ��Ӧ���ɵ��������ƣ���ȷ�������� g��
| �¶ȡ� | 0 | 10 | 20 | 30 | 40 | 60 | 80 | |
| �ܽ��/g | Na2CO3 | 7.00 | 12.5 | 21.5 | 39.7 | 49.0 | 46.0 | 43.9 |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.3 | 38.4 | |
��̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ�ȣ���ش�
��1��10��ʱ��̼���Ʊ��Ȼ��Ƶ��ܽ��
��2������ͼ�����ݣ������̼��ԭ����
��3��80��ʱ����100gˮ���Ƴ�̼���Ƶı�����Һ�����������������ǣ���ȷ������0.1%��

