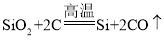��Ŀ����
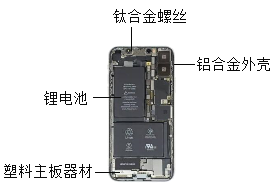
����Ŀ��ͭ�Ͻ���һ�ֳ��õĽ������ϡ�
(1)��ͭ(Cu2O)���ҹ��Ŵ���ȡ��ͭ��һ��ԭ�ϡ�Cu2O����Է�������Ϊ ��Cu2O��ͭԪ�غ���Ԫ�ص��������� ��
(2)���ⶨijͭþ�Ͻ�����(����Ԫ�غ��Բ���)����������ʵ�飺ȡͭþ�Ͻ�20g�����ձ�����200gϡ�����4�μ����ձ��У���ַ�Ӧ���ʣ������������¼���±�(Mg+H2SO4=MgSO4+H2��)��
�� �� | 1 | 2 | 3 | 4 |
����ϡ��������/g | 50 | 50 | 50 | 50 |
ʣ���������/g | 17.6 | m | 13.2 | 13.2 |
����㣺�ٱ���m= g����ͭþ�Ͻ���Ʒ��ͭ������= ��
������ϡ���������ʵ�����������(д���������)
���𰸡�(1)144 8��1��(2)��15.2 13.2 ��19.6%
��������
��1��Cu2O����Է�������=64��2+16=144��Cu2O��ͭԪ�غ���Ԫ�ص�������=��64��2����16=8:1��
��2���⣺���ɱ�����Ϣ��֪��ÿ����50gϡ���ᣬ������������20g-17.6g=2.4g�����ڶ��μ���50gϡ����ʱ��ʣ����������=17.6g-2.4g=15.2g���ɱ������ݿ�֪�������μ���50gϡ����ʱ�������С������С��2.4g��˵��þ�Ѿ���ȫ��Ӧ��ʣ��������������ͭ���������ʸ�ͭþ�Ͻ���Ʒ��ͭ������Ϊ13.2g��
�ڷ����������ݿ�֪,��50gϡ������ȫ��Ӧ����þ������Ϊ2.4g��
�裺��2.4gþ��Ӧ��ϡ���������ʵ�����Ϊx

![]()
x=9.8g
ϡ���������ʵ���������Ϊ:![]()

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�