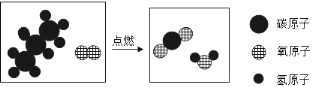
��Ŀ����
����Ŀ��ijͬѧ�����ճ������Ʋ⣬��ȼ��ȼ�տ������������¶��йأ���Ʋ��������ʵ�������֤��
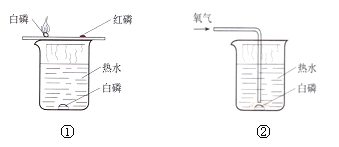
(1)����ȼ�յĻ�ѧ����ʽΪ__________________________________��
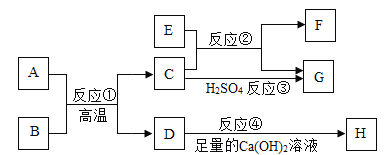
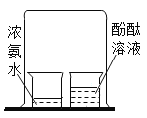
(2)ͼ����ͭƬ�ϵİ���ȼ�ն�ˮ�еİ��ײ�ȼ�գ�֤���˿�ȼ��ȼ�ձ���Ҫ________��ͨ����ͼ����_________________�İ���ͼ����ˮ�еİ���ͨ��������ȼ������ʵ������Ƚϣ��ܵó�ͬ�����۵ġ�
(3)ͼ���У�ͭƬ�ϵİ���ȼ�ն����ײ�ȼ�գ�֤���˺����Ż��Ȱ���____��ͬʱҲ֤���˿�ȼ��ȼ�ջ���Ҫ____________________��
(4)ʵ�����а��ױ�����ˮ�У��ƻ�ȼ�յ�������____________________��
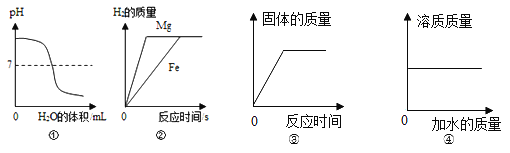
A �����ȼ�� B �����������Ӵ� C ʹ�¶Ƚ����Ż������
���𰸡�4P+5O2![]() 2P2O5 ���� ˮ�� �� �¶ȴﵽ��ȼ����Ż�� BC
2P2O5 ���� ˮ�� �� �¶ȴﵽ��ȼ����Ż�� BC
��������
��1������ȼ�յĻ�ѧ����ʽΪ 4P+5O2![]() 2P2O5��
2P2O5��
��2��ͼ����ͭƬ�ϵİ��Ӵ�����ȼ�ն�ˮ�еİ��ײ��Ӵ�������ȼ�գ�֤���˿�ȼ��ȼ�ձ���Ҫ������ͨ����ͼ����ˮ�а��ײ�ȼ�պ�ͼ����ˮ�еİ���ͨ��������ȼ�ա���ʵ������Ƚϣ�Ҳ�ܵó���ȼ��ȼ�ձ���Ҫ������
��3��ͼ���У�ͭƬ�ϵĺ��ס������Ӵ���������ͭƬ�ϵİ���ȼ�ն����ײ�ȼ�գ�֤���˺����Ż��Ȱ��ĸߣ�ͬʱҲ֤���˿�ȼ��ȼ�ջ���Ҫ�¶ȴﵽ��ȼ����Ż�㣻
��4�����ױ�����ˮ��һ�������ʹ���������������һ����Ҳ�ɱ������¶����Ż�����¡���ѡBC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ʵ������һƿ��ǩ�������ɫ��Һ������ͼ��ʾ����ʦҪ��ͬѧ�ǽ���̽������ƿ��Һ������ʲô��Һ��
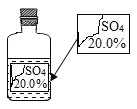
��������룩 ��ʾ����ƿ��ɫ��Һֻ��������������Һ�е�һ�֣�������þ��Һ������������Һ����������Һ�����������Һ
���������ϣ�
A�������£�������ʵ��ܽ�����£�
���� | MgSO4 | Na2SO4 | H2SO4 | (NH4)2SO4 |
�ܽ�� | 35.1g | 19.5g | ��ˮ����Ȼ��� | 75.4g |
B��MgSO4��(NH4)2SO4��ˮ��Һ������
��ش�
1.����ɫ��Һһ�����е�������_________��
��ʵ��̽������ҩƷ����ѡ��
2.ͨ���������ϣ�������ʵ��ܽ�ȱ�����С��ͬѧ��Ϊ����______������ţ���������ԭ����_____________________________________________��
3.Ϊȷ���������ֲ����Ƿ���ȷ��С��ͬѧ���ʵ���������̽����
ʵ����� | ʵ������ | ʵ����� |
ȡ����Һ�������Թ��У������еμӼ���_____��Һ | ��Һ���а�ɫ�������� | ����_____������ţ����� |
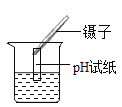
���ò�����պȡ����ԭ��Һ����pH��ֽ�ϣ�������ɫ������ |
| ���������� |
С��ͬѧ��ΪС��ʵ��������Ľ��۲���ȷ������������_______________��
����д��ʵ���������Ӧ�Ļ�ѧ����ʽ_______________________________��
����Ŀ��������ʵ������Ľ��ʹ������
A | B | C | D | |
ʵ������ |
��ɨ������������õķ���ͼ�� |
Ʒ�����ˮ�к�����Һ����ɫ |
ˮ�����ڲ�ͬ�¶��µ��˶���� |
50mLˮ��50mL�ƾ���Ϻ�����С��100mL |
���� | ���ӵ��������������С | �������Dz��ϵ��˶��ŵ� | ����ʱ���Ӳ��˶�������ʱ���Ӳ��˶� | �����п�϶ |
A.AB.BC.CD.D