��Ŀ����
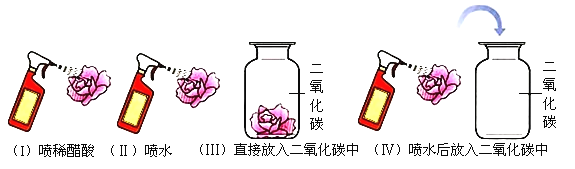
����Ŀ��С����ͼʾװ�ý���ʵ�顣��֪��2NaOH+SO2=Na2SO3+H2O���ٹر�K������������ȼ��ۺ�Ѹ����������������ȴ�����£�����ͷ�ι��е�����NaOH��Һ����ƿ�У���ʹ��Ӧ��֣��۴�K���۲����е���������˵����ȷ���ǣ�������
A. ����![]() �У���ȼ�ճʵ���ɫ���棬ƿ����ѹ��С
�У���ȼ�ճʵ���ɫ���棬ƿ����ѹ��С
B. ����![]() �У���װ���ڵ���ѹ��С��ֱ�������ѹ���
�У���װ���ڵ���ѹ��С��ֱ�������ѹ���
C. ����![]() �У����еij����ܿڴ�ð���ݣ�ˮ���������
�У����еij����ܿڴ�ð���ݣ�ˮ���������
D. ����![]() �У���װ���ڵ���ѹ����ֱ�������ѹ���
�У���װ���ڵ���ѹ����ֱ�������ѹ���
���𰸡�D
��������
A���������ڿ�����ȼ�գ�����ʵ���ɫ�����������������˶����������壬ƿ����ѹ���䣬�ʴ���
B������NaOH��Һ����ƿ����NaOH���������Ӧ�����������ƣ���������������٣�ѹǿ��С��С�ڴ���ѹ���ʴ���
C�����д�K��������ѹ����ƿ����ѹ�����еij����ܿڴ�ð���ݣ���ˮ���ᵹ������ף��ʴ���
D��������У���K������ͨ���ҽ���ף���װ���ڵ���ѹ����ֱ�������ѹ��ȣ���D�ԡ�
��ѡ��D��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�����Ŀ��ʵ���ҳ��õĸ������Ũ���ᡢ��ʯ��(CaO��NaOH�Ĺ�������)�ȣ������ڳ�ʪ�Ŀ������ױ��ʡ�ij��ѧ��ȤС���ʵ������һƿ���õļ�ʯ��չ��̽����
[��������]�ټ�ʯ�������տ����е�ˮ�����Ͷ�����̼ ���Ȼ�����Һ������,̼������Һ�ʼ��� ��̼���ƺ�������������ˮ�¶ȱ仯������ ��Ca(OH)2�ֽ��¶�Ϊ580�棬CaCO3�ֽ��¶�Ϊ825�棬Na2CO3�ķֽ��¶�Ϊ1744�档
[�������]��ʯ���Ƿ���ʣ���ɷֿ�������Щ��
[���в���]����û�б��ʣ���ʯ����ˮ�����ã��ɷ�ֻ��CaO��NaOH��
�������ʣ��ü�ʯ���п��ܺ���CaO��NaOH��Ca(OH)2��Na2CO3��CaCO3�е����ֻ��������ϡ�
[ʵ�����]
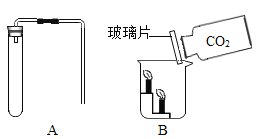
(1)��֤��ʯ���Ƿ���ʣ�ͼ�е�BΪ����װ�ã������ڹ��������������塣����A��Bװ�ü����ʯ���Ƿ���ʣ�ȡ��������ˮ����ͭ�ͼ�ʯ����Ʒ�ֱ�װ��A��B�У����Ӻ�A��Bװ�ã��� ____(����c������d��)����B�л���ͨ��ˮ�������۲�Aװ���е�����Ϊ____��֤����ʯ���ѱ��ʡ�
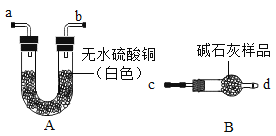
(2)��֤��ʯ���Ƿ���ȫ���ʣ�ȡ������ʯ����Ʒ�����Թ��У�������������ˮʹ�����ܽ⣬��Һ����ǣ����ִ����Թ���ڣ��¶������Ա仯��֤����ʯ������ȫ���ʡ�����Ʒ�ɷ������___�ֿ�����(������)��
(3)Ϊ��һ��ȷ����ʯ����Ʒ�ijɷ֣���С�����ʵ�鲢��¼���£�
ʵ���� | ʵ����� | ʵ��Ŀ�ġ���������� | ʵ����� |
ʵ��һ | ��ȡ������Ʒ���Թ��У�������������ˮʹ�����ܽ⣻ �ڹ��ˣ��õ�����A����ҺB��������ҺB�м�������CaCl2��Һ�����ã� ��________�� | �����۵���ҪĿ���ǣ�___ �����ܵ�����____ | ��Ʒ��һ����Ca(OH)2 |
ʵ��� | ��ȡ������Ʒ50g��������600���ڣ��������������ٷ����仯����ȴ������� �ڽ�����ʣ����������850���ڷ������ȣ���ȴ������� | �������гƵù�������Ϊ45.5g�������ڹ��������ޱ仯�� | ��Ʒ��һ��û��_____(�ѧʽ) |
[̽������] ͨ������̽���������֪��ʯ����Ʒ�ijɷ���______(�ѧʽ)�������ε���������Ϊ_____��
[��˼������] ͨ��̽��������֪���ü�ʯ�ұ��ʵĻ�ѧ��Ӧ���̣����������εĻ�ѧ����ʽΪ______�������˼�ʯ��Ҫ�ܷⱣ���ԭ��
����Ŀ���Ͻ������ܶ࣬��;�dz��㷺����ͭ��ͭ��п�ĺϽ�������������������͵���������ȣ���ѧ��ȤС���ͬѧ���ⶨʵ������ijͭ��Ʒ��ͭ�����������������ǻ�ͭ�е��������ʣ�������������ǵ�̽��������
����10g��ĩ״��ͭ��Ʒ�����ձ��У���ȡ45mLϡ��������μӵ����У�ÿ�γ�ַ�Ӧ�ⶨ����������������ʵ�����������
��һ�� | �ڶ��� | ������ | |
����ϡ����������ml�� | 15 | 15 | 15 |
����������������g�� | 0.04 | m | 0.02 |
����
��1��m����ֵ ��
��2���˻�ͭ��Ʒ��ͭ�����������Ƕ��٣���д��������̣�
����Ŀ��ʵ���ҳ��ù���������Һ�Ͷ������̣���������ȡ������
��������⣩���������Ǵ������������̵������Է�Ӧ�����Ƿ���Ӱ�죿
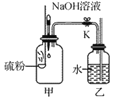
�����ʵ�飩��1��ʵ��װ����ͼ��ʾ��
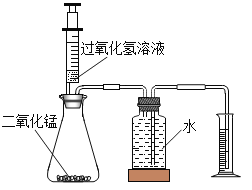
��2��ʵ���¼��ÿ����30����10%�Ĺ���������Һ�����ò�ͬ���������̷�ĩ����������ʵ�飬�ⶨ������ݼ�¼���±��У�
ʵ����� | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
��������������g�� | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
�������� |
����������1������ʵ��Ӧ�òⶨ��������������������_____________��
��2��10��ʵ����ÿ����30����10%�Ĺ���������Һ����Ŀ����_____________��
p>��3������2��ʵ��ȵ�3���������������������______________��������������С������˵������������Խ�࣬��ӦԽ�죮��ʵ�������������ʵ��֤������1��ʵ������7��ʵ���й�������ķֽ��������μӿ죬��7��ʵ������10��ʵ������¼�������������������Բ��죮
�����ۣ�__________________��