��Ŀ����
 С��ͬѧ����������ʱ��С�Ľ�һƿ�״������ڻ�¯�Ե�һ�Ѳ�ľ���ϣ������д������������ɣ���������Ȥ��������������ѧϰС���ͬѧ���Բ�ľ�ҵijɷֽ���̽����
С��ͬѧ����������ʱ��С�Ľ�һƿ�״������ڻ�¯�Ե�һ�Ѳ�ľ���ϣ������д������������ɣ���������Ȥ��������������ѧϰС���ͬѧ���Բ�ľ�ҵijɷֽ���̽����
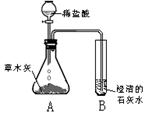
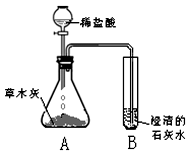
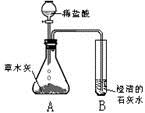
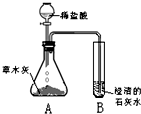
[̽���һ]������ѧ�����֪ʶ��ͬѧ�Dz����ľ���к��������ᷴӦ����������������ʣ����ǣ���Ʋ���������ͼ��ʾ��ʵ�飮�۲쵽��ƿA���д���������ð�����Թ�B�г����ʯ��ˮ����ǣ���������ʵ�������ƶϣ������������к���______���Թ�B���йط�Ӧ�Ļ�ѧ����ʽΪ______��
[̽�����]����ժҪ��ľ���е���Ҫ�ɷ���̼��أ�̼��ص�ˮ��Һ��̼������Һ��������ƣ�
��1����̼��ص�ˮ��Һ�е����̪��Һ����Һ��______ɫ��
��2������д��������̽���һ���У���ƿA�ڷ�����һ����Ӧ�Ļ�ѧ����ʽ��______��
��3��ijͬѧ���ⶨ��Щ��ľ����̼��صĺ���������ƽ��ȡ69g�������ձ��У�����100gϡ����ǡ����ȫ��Ӧ����ַ�Ӧ�Ƶû�����������Ϊ164.6g�����Զ�����̼���ܽ��������Ӱ�죩������㣺
�ٷ�Ӧ�����в����Ķ�����̼����Ϊ______g��
�ڸò�ľ��������̼��ص�����������
��������ʵ�������õ�ϡ����100g����Ҫ������������Ϊ14.6%��������ٿˣ�
�⣺[̽���һ]�����ʯ��ˮ�����˵����Ӧ�����˶�����̼���÷�Ӧ�ж�����̼���������Ʒ�Ӧ���ɲ����Ե�̼��Ƴ�����ˮ��
�ʴ�Ϊ��������̼����CO2���� Ca��OH��2+CO2�TCaCO3��+H2O��
[̽�����]
��1��̼������Һ�Լ��ԣ���̼��ص�ˮ��Һ��̼������Һ��������ƣ�����̼�����ҺҲ�Լ��ԣ���ʹ��̪��Һ��죻
�ʴ�Ϊ���죻
��2���������֪����ľ���е���Ҫ�ɷ���̼��أ���ϡ���ᷴӦʱ���ֻ�����������ɷ������Ȼ��غ�̼�ᣬ̼��ȶ����ֽ�����̼�
�ʴ�Ϊ��K2CO3+2HCl=2KCl+H2O+CO2����
��3���ٸ��������غ㶨�ɿ�֪������̼��������69g+100g-164.6g=4.4g��
�ʴ�Ϊ��4.4 g��
�ڽ⣺��������K2CO3������Ϊx������HCl������Ϊy
K2CO3+2HCl=2KCl+H2O+CO2��
138 73 44
x y 4.4 g
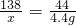
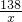 =
=
��ã�x=13.8 g y=7.3 g
��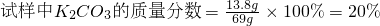 ��
��
������Ҫ������������Ϊ14.6%����������ΪZ��
����Z��14.6%=7.3g��
��ã�Z=50g
�𣺷�Ӧ�����в����Ķ�����̼����Ϊ4.4g�� ��ľ��������̼��ص�����������20%�� ����ʵ�������õ�ϡ����100g����Ҫ������������Ϊ14.6%������50g��
������[̽���һ]���ݶ�����̼��ʹ�����ʯ��ˮ����ǽ��з����������ݷ�Ӧԭ��д������ʽ��
[̽�����]��1������̼��ص�ˮ��Һ��̼������Һ��������ƣ�̼������Һ�Լ��Է������
��2��̼�����ϡ����ķ�Ӧ���ڸ��ֽⷴӦ�����ݸ��ֽⷴӦ�ķ�Ӧ�ص���д����ʽ��
��3�����ȸ��������غ㶨�����������̼��������Ȼ�����ݶ�����̼�����������̼�����ϡ���ᷴӦ����ʽ����̼��ص������������������̼��ص��������������ݶ�����̼����������μӷ�Ӧ���������ʵ��������ٸ������ʵ�����=����������������Һ������������
���������ⴴ�������龳��������̼���κͶ�����̼�����ʼ�����ʽ����д�������ݷ���ʽ���м��㣬�ܺܺõ�����ѧ��������������������
�ʴ�Ϊ��������̼����CO2���� Ca��OH��2+CO2�TCaCO3��+H2O��
[̽�����]
��1��̼������Һ�Լ��ԣ���̼��ص�ˮ��Һ��̼������Һ��������ƣ�����̼�����ҺҲ�Լ��ԣ���ʹ��̪��Һ��죻
�ʴ�Ϊ���죻
��2���������֪����ľ���е���Ҫ�ɷ���̼��أ���ϡ���ᷴӦʱ���ֻ�����������ɷ������Ȼ��غ�̼�ᣬ̼��ȶ����ֽ�����̼�
�ʴ�Ϊ��K2CO3+2HCl=2KCl+H2O+CO2����
��3���ٸ��������غ㶨�ɿ�֪������̼��������69g+100g-164.6g=4.4g��
�ʴ�Ϊ��4.4 g��
�ڽ⣺��������K2CO3������Ϊx������HCl������Ϊy
K2CO3+2HCl=2KCl+H2O+CO2��
138 73 44
x y 4.4 g
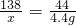
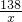 =
=
��ã�x=13.8 g y=7.3 g
��
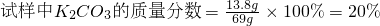 ��
��������Ҫ������������Ϊ14.6%����������ΪZ��
����Z��14.6%=7.3g��
��ã�Z=50g
�𣺷�Ӧ�����в����Ķ�����̼����Ϊ4.4g�� ��ľ��������̼��ص�����������20%�� ����ʵ�������õ�ϡ����100g����Ҫ������������Ϊ14.6%������50g��
������[̽���һ]���ݶ�����̼��ʹ�����ʯ��ˮ����ǽ��з����������ݷ�Ӧԭ��д������ʽ��
[̽�����]��1������̼��ص�ˮ��Һ��̼������Һ��������ƣ�̼������Һ�Լ��Է������
��2��̼�����ϡ����ķ�Ӧ���ڸ��ֽⷴӦ�����ݸ��ֽⷴӦ�ķ�Ӧ�ص���д����ʽ��
��3�����ȸ��������غ㶨�����������̼��������Ȼ�����ݶ�����̼�����������̼�����ϡ���ᷴӦ����ʽ����̼��ص������������������̼��ص��������������ݶ�����̼����������μӷ�Ӧ���������ʵ��������ٸ������ʵ�����=����������������Һ������������
���������ⴴ�������龳��������̼���κͶ�����̼�����ʼ�����ʽ����д�������ݷ���ʽ���м��㣬�ܺܺõ�����ѧ��������������������

��ϰ��ϵ�д�
�����Ŀ
 ��2012?�γ�ģ�⣩С��ͬѧ����������ʱ��С�Ľ�һƿ�״������ڻ�¯�Ե�һ�Ѳ�ľ���ϣ������д������������ɣ���������Ȥ��������������ѧϰС���ͬѧ���Բ�ľ�ҵijɷֽ���̽����
��2012?�γ�ģ�⣩С��ͬѧ����������ʱ��С�Ľ�һƿ�״������ڻ�¯�Ե�һ�Ѳ�ľ���ϣ������д������������ɣ���������Ȥ��������������ѧϰС���ͬѧ���Բ�ľ�ҵijɷֽ���̽����