��Ŀ����
 ѧϰ�����ѧϰ�����dzɲŵıر�������������ղ�ͬ��ѧϰ��������ȡ���°빦����Ч������ͼ��ʾ��С��ͬѧ��ʢ�и�����ļ��Թܺ���������Թܲ���ʢ��80����ձ���ס����Թܾ����������ܷ⣩����һ����ּ��Թ��е�
ѧϰ�����ѧϰ�����dzɲŵıر�������������ղ�ͬ��ѧϰ��������ȡ���°빦����Ч������ͼ��ʾ��С��ͬѧ��ʢ�и�����ļ��Թܺ���������Թܲ���ʢ��80����ձ���ס����Թܾ����������ܷ⣩����һ����ּ��Թ��е�����ȼ�գ����Թ��еĺ���û��ȼ�գ������С��ͬѧ��ʵ�����Աȵó���ȼ��ȼ�����������֮һ��
��ȼ���¶ȴﵽ�Ż��
��ȼ���¶ȴﵽ�Ż��
����������������ȼ�յ��������Ա������Թ��е�����������������ͬ����з�����
����⣺��������ȼ�յ�������֪��A��B�Թ������¶���ͬ��ͬ���������Ӵ���A�Թ��еİ�����ȼ�գ�B�Թ��еĺ��ײ���ȼ�գ�˵��B�Թ��еĺ���û�дﵽ�Ż�㣮
�ʴ�Ϊ����ȼ���¶ȴﵽ�Ż�㣮
�ʴ�Ϊ����ȼ���¶ȴﵽ�Ż�㣮
���������ȼ�յ������ǽ���Ĺؼ���

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
ѧϰ�����ѧϰ�����dzɲŵıر�������������ղ�ͬ��ѧϰ��������ȡ���°빦����Ч�����������·�������������⣺
��1�����෨����������4������
A��ZnO��MgO��CO2��Na2O�������������� B��Cu��N2��O2��Cl2
C��KNO3��NaHCO3��KClO3��Fe��OH��3�� D��H2SO4��H2O��HCl��HNO3
�밴Ҫ����д�±��հף���д���ʵĻ�ѧʽ��������
| \ | A | B | C | D |
| ����� | ���������� | ________ | �� | ________ |
| �����ڸ��������� | ________ | Cu | ________ | H2O |
A��ͨ����CO2+H2O�TH2CO3��6CO2+6H2O
 C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ�����________��
C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ�����________��B����ͼ��ʾ��С��ͬѧ��ʢ�и�����ļ��Թܺ���������Թܲ���ʢ��80����ձ���ס����Թܾ����������ܷ⣩����һ����ּ��Թ��еİ���ȼ�գ����Թ��еĺ���û��ȼ�գ������С��ͬѧ��ʵ�����Աȵó���ȼ��ȼ�����������֮һ��________��

��3�����������
С��ͬѧͨ���Դ�����ѧ��Ӧ������������Һ����������Һ��˫��ˮ�ֽ⣨����ͼ����Ӧǰ������ʵ������ܺ͵IJⶨ���ó��μӻ�ѧ��Ӧ�ĸ����ʵ������ܺ�________������ڡ�����С�ڡ����ڡ�����Ӧ�����ɵĸ������ʵ������ܺͣ�

����С���ó��Ľ��ۣ����Ƴ�7.9g������ؼ���һ���ʣ���������Ϊ7.5g����Ӧ��������������Ϊ________g��
ѧϰ�����ѧϰ�����dzɲŵıر�������������ղ�ͬ��ѧϰ��������ȡ���°빦����Ч�����������·�������������⣺
��1�����෨����������4������
A��ZnO��MgO��CO2��Na2O B��Cu��N2��O2��Cl2
C��KNO3��NaHCO3��KClO3��Fe��OH��3 D��H2SO4��H2O��HCl��HNO3
�밴Ҫ����д�±��հף���д���ʵĻ�ѧʽ��������
��2���Աȷ���
A��ͨ����CO2+H2O�TH2CO3��6CO2+6H2O C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ����� ��
C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ����� ��
B����ͼ��ʾ��С��ͬѧ��ʢ�и�����ļ��Թܺ���������Թܲ���ʢ��80����ձ���ס����Թܾ����������ܷ⣩����һ����ּ��Թ��еİ���ȼ�գ����Թ��еĺ���û��ȼ�գ������С��ͬѧ��ʵ�����Աȵó���ȼ��ȼ�����������֮һ�� ��

��3�����������
С��ͬѧͨ���Դ�����ѧ��Ӧ������������Һ����������Һ��˫��ˮ�ֽ⣨����ͼ����Ӧǰ������ʵ������ܺ͵IJⶨ���ó��μӻ�ѧ��Ӧ�ĸ����ʵ������ܺ� ������ڡ�����С�ڡ����ڡ�����Ӧ�����ɵĸ������ʵ������ܺͣ�
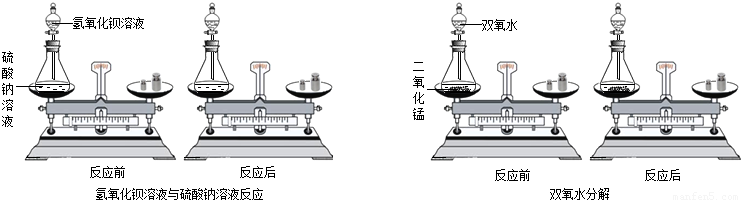
����С���ó��Ľ��ۣ����Ƴ�7.9g������ؼ���һ���ʣ���������Ϊ7.5g����Ӧ��������������Ϊ g��
��1�����෨����������4������
A��ZnO��MgO��CO2��Na2O B��Cu��N2��O2��Cl2
C��KNO3��NaHCO3��KClO3��Fe��OH��3 D��H2SO4��H2O��HCl��HNO3
�밴Ҫ����д�±��հף���д���ʵĻ�ѧʽ��������
| \ | A | B | C | D |
| ����� | ���������� | �� | ||
| �����ڸ��������� | Cu | H2O |
A��ͨ����CO2+H2O�TH2CO3��6CO2+6H2O
 C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ����� ��
C6H12O6+6O2������ѧ��Ӧ�ĶԱȣ����ܵó��Ľ����� ��B����ͼ��ʾ��С��ͬѧ��ʢ�и�����ļ��Թܺ���������Թܲ���ʢ��80����ձ���ס����Թܾ����������ܷ⣩����һ����ּ��Թ��еİ���ȼ�գ����Թ��еĺ���û��ȼ�գ������С��ͬѧ��ʵ�����Աȵó���ȼ��ȼ�����������֮һ�� ��

��3�����������
С��ͬѧͨ���Դ�����ѧ��Ӧ������������Һ����������Һ��˫��ˮ�ֽ⣨����ͼ����Ӧǰ������ʵ������ܺ͵IJⶨ���ó��μӻ�ѧ��Ӧ�ĸ����ʵ������ܺ� ������ڡ�����С�ڡ����ڡ�����Ӧ�����ɵĸ������ʵ������ܺͣ�

����С���ó��Ľ��ۣ����Ƴ�7.9g������ؼ���һ���ʣ���������Ϊ7.5g����Ӧ��������������Ϊ g��


