��Ŀ����
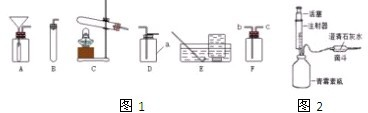
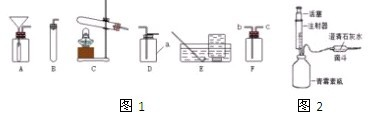
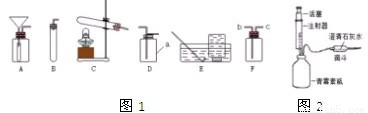
 ��ͼ��ʵ�����ø��������������װ��ͼ��ָ��ͼ�е���������˵���ɴ˿�����ɵĺ����
��ͼ��ʵ�����ø��������������װ��ͼ��ָ��ͼ�е���������˵���ɴ˿�����ɵĺ������1������
�Թ��е�����ҩƷ���ȴ�̫��
�Թ��е�����ҩƷ���ȴ�̫��
�����������ʱ��ȼ��������װ�ñ�ը
����ʱ��ȼ��������װ�ñ�ը
����2������
�Թܿ�������б
�Թܿ�������б
�������������ˮ������������Թܵײ�����ʹ�ȵ��Թ�ը��
������ˮ������������Թܵײ�����ʹ�ȵ��Թ�ը��
����3������
����ǰ���ܿڲ�Ӧ���뼯��ƿ��
����ǰ���ܿڲ�Ӧ���뼯��ƿ��
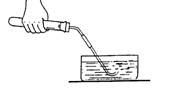
���������ʹ�ռ�����������п���������
��ʹ�ռ�����������п���������
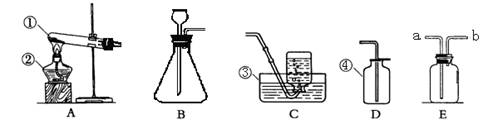
�������������ø��������ȡ�����ķ�Ӧԭ���Ͳ���ʱ��Ӧע������������������������IJ�������������⣮
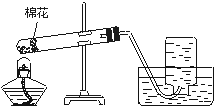
����⣺�۲�ͼʾ��֪���Թ��е�������ҩƷ���ȴ�̫��������ʱ�п�����ȼ��������װ�ñ�ը���Թܿ�������б����ʹ����������ˮ�������Թܵײ���ʹ�ȵ��Թ�ը�ѣ�ͼʾ�еĻ�û�м��ȣ����Ե��ܿڲ�Ӧ���뼯��ƿ�ڣ������ʹ�ռ�����������п�����������
�ʴ�Ϊ����1���Թ��е�����ҩƷ���ȴ�̫��������ʱ��ȼ��������װ�ñ�ը��
��2���Թܿ�������б��������ˮ������������Թܵײ�����ʹ�ȵ��Թ�ը�ѣ�
��3������ǰ���ܿڲ�Ӧ���뼯��ƿ�ڣ���ʹ�ռ�����������п�����������
�ʴ�Ϊ����1���Թ��е�����ҩƷ���ȴ�̫��������ʱ��ȼ��������װ�ñ�ը��
��2���Թܿ�������б��������ˮ������������Թܵײ�����ʹ�ȵ��Թ�ը�ѣ�
��3������ǰ���ܿڲ�Ӧ���뼯��ƿ�ڣ���ʹ�ռ�����������п�����������
�������ø���������������dz��л�ѧ�е���Ҫʵ��֮һ����֮��ص����⣬ͬѧ��һ��Ҫ��Ȼ���ģ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ