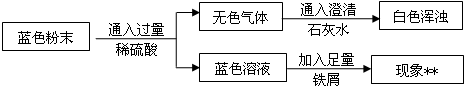
��Ŀ����
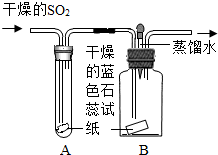 ij�о���ѧϰС��ԡ�SO2�ܷ���H2O��Ӧ�����ᡱ����̽��������������ǵ�̽��������ش��й����⣮
ij�о���ѧϰС��ԡ�SO2�ܷ���H2O��Ӧ�����ᡱ����̽��������������ǵ�̽��������ش��й����⣮
��1���������ϣ���SO2��������һ����ɫ���壬������ˮ��������ʹ��ɫʯ����ֽ��ɺ�ɫ����SO2�ж���
��2��������裺SO2����H2O��Ӧ�����ᣮ
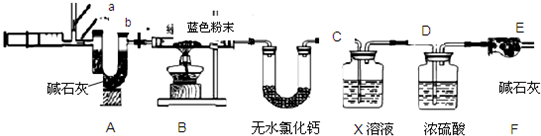
��3��ʵ��̽�����������ͼ��ʾװ�ý���ʵ�飮
��ʵ������У�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��Aװ�õ�������________��
����ͨ��SO2֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯���˲�������Ŀ����________������SO2ͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵��________���˹����з�Ӧ�Ļ�ѧ����ʽΪ________��
��4�����ۣ�ԭ���������
��5����˼�����ۣ���ʵ�鷽���У���һ�����Ե���©�����������ָ������֮��________��
��6����չ̽�������о���ѧϰС��ȡ�ս������᳧�����������в���SO2����������ˮ���вⶨ��ÿ�������Ӳ�һ��pH�����������±���ʾ��
| �ⶨʱ�� | 5��05 | 5��10 | 5��15 | 5��20 | 5��25 | 5��30 | 5��35 |
| pH | 4.95 | 4.94 | 4.94 | 4.88 | 4.86 | 4.85 | 4.85 |
�⣺�����SO2ͨ��Aװ�ã�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��˵����SO2����ʹ��ɫʯ����ֽ��ɫ������ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯��˵��ˮ����ʹ��ɫʯ����ֽ��ɫ������SO2ͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵�� SO2��ˮ��Ӧ�����ᣬ�˹����з�Ӧ�Ļ�ѧ����ʽΪ��SO2+H2O=H2SO3��SO2���ж������壬�������β���������ӱ����п��Կ���������ʱ��ı仯����ˮ����������ǿ������������������Ը�ǿ�����ᣬ���ԱȽϣ�H2SO4��H2SO3��H2CO3
�𣺣�3����֤��SO2����ʹ��ɫʯ����ֽ��ɫ��֤��ˮ����ʹ��ɫʯ����ֽ��ɫ��SO2��ˮ��Ӧ�����ᡢSO2+H2O=H2SO3
��5��β��û�д�������Ӧ�ӽ�һ��β������װ�ã�
��6������ʱ��ı仯����ˮ����������ǿ��H2SO3������е�O2��Ӧ������H2SO4��H2SO4��H2SO3��H2CO3��
����������ע�����ʵ���˳���磺������ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯��˵��ˮ����ʹ��ɫʯ����ֽ��ɫ��Ȼ����SO2ͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵�� SO2��ˮ��Ӧ�����ᣬ�˹����з�Ӧ�Ļ�ѧ����ʽΪ��SO2+H2O=H2SO3��Ȼ�������ˮ����������ǿ���������������Ը�ǿ�����ᣮ���Ƚ�����ǿ����H2SO4��H2SO3��H2CO3
����������̽����SO2�ܷ���H2O��Ӧ�����ᡱ���й����⣬ע��ʵ��װ�õĴ������̣������SO2ͨ��Aװ�ã�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��˵����SO2����ʹ��ɫʯ����ֽ��ɫ��Ҫע���������ʱ��ı仯����ˮ����������ǿ��ԭ��
�𣺣�3����֤��SO2����ʹ��ɫʯ����ֽ��ɫ��֤��ˮ����ʹ��ɫʯ����ֽ��ɫ��SO2��ˮ��Ӧ�����ᡢSO2+H2O=H2SO3
��5��β��û�д�������Ӧ�ӽ�һ��β������װ�ã�
��6������ʱ��ı仯����ˮ����������ǿ��H2SO3������е�O2��Ӧ������H2SO4��H2SO4��H2SO3��H2CO3��
����������ע�����ʵ���˳���磺������ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯��˵��ˮ����ʹ��ɫʯ����ֽ��ɫ��Ȼ����SO2ͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵�� SO2��ˮ��Ӧ�����ᣬ�˹����з�Ӧ�Ļ�ѧ����ʽΪ��SO2+H2O=H2SO3��Ȼ�������ˮ����������ǿ���������������Ը�ǿ�����ᣮ���Ƚ�����ǿ����H2SO4��H2SO3��H2CO3
����������̽����SO2�ܷ���H2O��Ӧ�����ᡱ���й����⣬ע��ʵ��װ�õĴ������̣������SO2ͨ��Aװ�ã�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��˵����SO2����ʹ��ɫʯ����ֽ��ɫ��Ҫע���������ʱ��ı仯����ˮ����������ǿ��ԭ��

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ