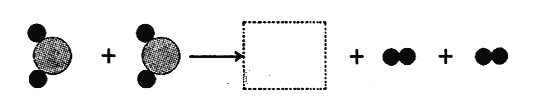
��Ŀ����
����Ŀ����ͼ��ij��ͬѧ��֤�����غ㶨��ʵ���Ƭ�Ρ�
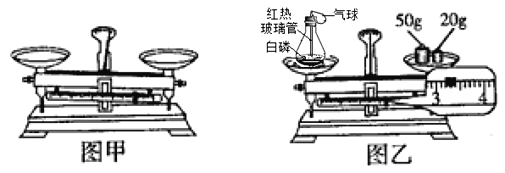
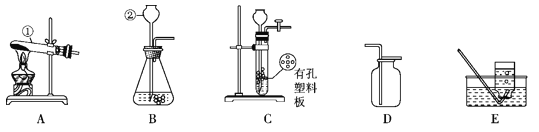
��1����ͼ����ʾ����ƽ������ƽ�⡣Ҫʹ����ƽƽ�⣬Ӧ��ȡ�Ĵ�ʩ________��
��2��С���ͼ����ʾװ�ý���ʵ�顣��Ӧǰ�Ƶ�������װ�ü�����������Ϊ_______g��Ȼ����ȼ���ף���ַ�Ӧ����ȴ�����£���֤�������غ㶨�ɵ�������_______��������Ӧ�Ļ�ѧ����ʽΪ_____��
��3�����жԸ�ʵ������ۣ�����Ϊ��������____ ������ţ���
A�����������Ķ��٣����֤�������غ㶨�ɵĽ��۲���Ӱ��
B��ȥ��������ƽ�Խ�����ƽ��
C��δ����ȴ�����¾ͽ��г�������ƽ���ܻ�ʧȥƽ��
���𰸡��Ƚ�������㣬�ٵ���ƽ����ĸ 73.2 ��ƽ�Ա���ƽ�⣨����������Ϊ73.2g�� 4P + 5O2 ![]() 2P2O5 C
2P2O5 C
��������
��1��������ƽƽ��ķ������Ƚ�������㣬�ٵ���ƽ����ĸ�������Ƚ�������㣬�ٵ���ƽ����ĸ��
��2����ͼ�ҿ��Կ������������Ϊ70g������Ŀ̶�Ϊ3.2g�����ʵ�������Ϊ73.2g������73.2����Ӧ�����У���ƽ��ָ��һֱָ�ڷֶ��̵��м�����ʾ��ƽ�Ա���ƽ�⣨����������Ϊ73.2g����������ƽ�Ա���ƽ�⣨����������Ϊ73.2g����ͼ���еķ�Ӧ�ǰ��Ϳ����е�����ȼ���������������ף��ʷ�Ӧ�Ļ�ѧ����ʽдΪ��4P+5O2 ![]() 2P2O5��
2P2O5��
��3��A���μӷ�Ӧ�ĸ����������ܺ͵��ڷ�Ӧ�����ɵĸ����������ܺͣ����Ķ��ٲ���Խ��۲���Ӱ����ѡ�����B��ȥ����������������ɢ���������У�������������������ƽʧȥƽ�⣬ѡ�����C��δ����ȴ�����¾ͽ��г�������������һ���ĸ�������ʵ���������ƽ���ܻ�ʧȥƽ����ѡ����ȷ����ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ƾ�����ʵ�����г��ü���������ij��ȤС��ͬѧ�Ծƾ��ƽ�������̽����
�������о���ͬѧȡһ�������סһ��Ѹ��ƽ����ƾ��ƻ����У�1��2s��ȡ�����۲쵽λ�����ĵIJ���û�����Ա仯��˵�������¶���͡�
��1��д��̼��ȫȼ�յ����ֱ���ʽ������ű���ʽ��__________��
�������о�
��ͬѧ��ע������ȡ��о�����Ľ��紦���壬������������������������������������±�������ͬѧ���ø��´������ⶨ�ƾ��Ƶ�о�����Ľ��紦�¶ȣ����±�����
�������� | ��һ�� | �ڶ��� | ������ |
��о�����Ľ��紦 ����������� | 5.93% | 5.29% | 6.53% |
��о�����Ľ��紦�¶�/�� | 236 | 243 | 240 |
��2���ҡ���ͬѧ��ν��в�����Ŀ����_____��
��3�����ȼ����Ҫ��������ͺ�����14.0%���Ż����270�棬����ϱ�̸̸��ԡ���������Ĵ�û�����Ա仯������ʶ_____��
��4��������dz���ʱ������Ӱ�죬����ͬѧ���������Ũ�ȱ����ĵ�ʵ������Ũ��_____������ߡ��͡�����������ͬѧ����������������������ԭ������Ǣ�_____����_____��
����Ŀ����ѧ����С��ͬѧȡ8gʯ��ʯ����Ҫ�ɷ���CaC03����Ʒ���ձ��У����вⶨʵ�顣�ֽ�40gϡ���� ���Ĵμ����ձ��У���Ӧ�Ļ�ѧ����ʽΪ��CaC03+2HCl=CaCl2+C02��+H20��ʯ��ʯ���������ʲ�����ˮҲ�����뷴Ӧ����Ӧ���ɵ�CaCl2��ȫ���ܽ���ˮ�У�����ַ�Ӧ����й��������±���ʾ��
ʵ�� | ��1�� | ��2�� | ��3�� | ��4�� |
����ϡ���������/g | 10 | 10 | 10 | 10 |
�ձ���ʣ����������/g | 5.5 | 3.0 | m | 1.2 |
��1��8gʯ��ʯ��Ʒ�����ʵ�����Ϊ__________g��
��2���ϱ���m����ֵ��____________��
��3�����¶���ʯ��ʯ��Ʒ���Ƶ���ʯ�ң�CaO���Ͷ�����̼�������������ʯ��ʯ��Ʒ100kg�����ʲ���Ӧ����������������Ƶ������ƶ���____________kg?�����ݻ�ѧ����ʽ��ʽ���㣬ע���ʽ�Ĺ淶��