��Ŀ����
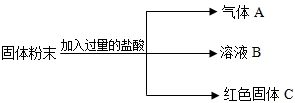
��һ�������ĩ��������CaCO3��Na2SO4��CuSO4��Na2CO3�е�һ�ֻ���֣�ijʵ��С�������ɳɷֽ���������̽�������˼���뽻������1�����������У�������ˮ����______��_______(�ѧʽ����ͬ)��
��2��������ˮ����ˮ��Һ����ɫ����______��_______��
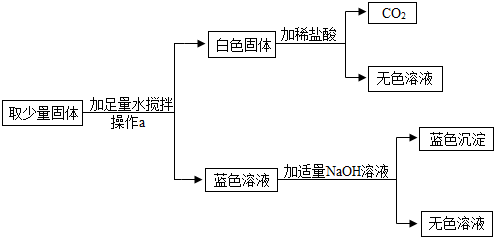
�����ϲ��ġ�Na2CO3��Һ��CuSO4��Һ����ܲ�����ɫ������
�����̽����ȥһ�������������ĩ��������ˮ�ܽ⡣���ܽ������һ��Ҫ�õ��IJ���������______��_______�Ͳ����������в�������������______��_______��
����������ۡ��������������õ���ɫ������Һ����ԭ�����ĩ��һ��û��____��_____��____��_____���ѧʽ����
��̽������չ��Ϊ��һ��ȷ��ԭ�����ĩ����ɣ���ʵ��С��ͬѧ����������ƽ��ȡ46.2g�÷�ĩ������ˮ���174.6g��Һ���������м���������������Ϊ8%��NaOH��Һ���������·�Ӧ��CuSO4+2NaOH = Na2SO4+Cu��OH��2������������������������NaOH��Һ�����Ĺ�ϵ��ͼ��ʾ����ͼ�ش𣺣��������С�����һλ��
��3�����������������ʱ����NaOH��Һ��������
��4��ͨ������ȷ��46.2g�����ĩ����ɡ����ش���������ƺ���������
��5������A����ʾ��Һ�����ʵ�����������

��1����CaCO3
��2����CuSO4 ���ձ� �ܽ��裨���ٹ�����ܽ⣩
��CaCO3 ��Na2CO3
��3���⣺��������������ʱ����NaOH������Ϊx��46.2g��ĩ�к���CuSO4������Ϊy����Na2SO4������Ϊz
CuSO4+2NaOH = Na2SO4+Cu��OH��2��
160 80 142 98
y x z 19.6g

x=16.0g������������������������������������������������������1��
��ôNaOH��Һ������= =200.0g����������������������������1��
=200.0g����������������������������1��
y=32.0g������������������������������������������������������1��
z=28.4g������������������������������������������������������1��
��4��Na2SO4������=46.2g-32g=14.2g������������������������������1��
��46.2g�����ĩ��14.2g Na2SO4��32.0g CuSO4��ɡ�
��5��Na2SO4����������= ��100%
��100%
=12.0%������������������������������������1��
���ԡ�����:
��
��2����CuSO4 ���ձ� �ܽ��裨���ٹ�����ܽ⣩
��CaCO3 ��Na2CO3
��3���⣺��������������ʱ����NaOH������Ϊx��46.2g��ĩ�к���CuSO4������Ϊy����Na2SO4������Ϊz
CuSO4+2NaOH = Na2SO4+Cu��OH��2��
160 80 142 98
y x z 19.6g

x=16.0g������������������������������������������������������1��
��ôNaOH��Һ������=
 =200.0g����������������������������1��
=200.0g����������������������������1��y=32.0g������������������������������������������������������1��
z=28.4g������������������������������������������������������1��
��4��Na2SO4������=46.2g-32g=14.2g������������������������������1��
��46.2g�����ĩ��14.2g Na2SO4��32.0g CuSO4��ɡ�
��5��Na2SO4����������=
 ��100%
��100%=12.0%������������������������������������1��
���ԡ�����:
��

��ϰ��ϵ�д�
�����Ŀ