��Ŀ����
����Ŀ��������dz��õ��մ���ϴҺ,С��ͬѧ��ijƷ�ƽ�������Ч�ɷּ��京�������о���
��1���������ϵ�֪:��������Ч�ɷ���HCl,HCl�ĺ�����ͨ����֪��������������NaHCO3��Һ���ⶨ,�����ɷ־������뷴Ӧ�������HCl��NaHCO3��Ӧ�Ļ�ѧ����ʽ:HCl+NaHCO3=NaCl+___+CO2����
��2����һ��ƿ�м���100 g��Ʒ�ƵĽ����,����μ�����ͬ����������NaHCO3��Һ,���ÿ����ƿ�з�Ӧ����Һ��������,���ݼ�¼����:
��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | |
����NaHCO3��Һ������/g | 40 | 40 | 40 | 40 | 40 |
��Ӧ����Һ��������/g | 138.9 | 177.8 | 216.7 | 255.6 | 295.6 |
�� ��________��ǡ����ȫ��Ӧ��
�� ��Ʒ�ƽ������HCl�����������Ƕ���?________
���𰸡�H2O �� 3.65%
��������
��1��HCl��NaHCO3��Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ��HCl+NaHCO3=NaCl+H2O+CO2����
��2���ٵ�һ�����Ĵμ���40g̼��������Һ��������1.1g�Ķ�����̼������μ���40g̼������û�в���������̼���ʵ��Ĵ�ǡ����ȫ��Ӧ��
����:��100 g��Ʒ�ƽ������HCl��������x��
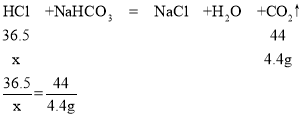
x=3.65 g
�������HCl������������![]() ��
��
��:��Ʒ�ƽ������HCl������������3.65%��