ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ Β―ι “œ÷”–¥σάμ ·ΓΔœΓΝρΥαΓΔœΓ―ΈΥαΓΔΥΪ―θΥ°ΓΔ ·»οΓΔΜπ≤ώΓΔΟόΜ®ΦΑ“‘œ¬“«Τς:

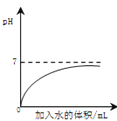
Θ®1Θ©»τ≤Ι≥δ“Μ÷÷“«ΤςΔΌ__________Θ®ΧνΟϊ≥ΤΘ©Θ§≤Δάϊ”Ο…œ ω“«ΤςΔΎ_____________Χν–ρΚ≈Θ©ΚΆ≤ΩΖ÷“©ΤΖΩ…÷Τ»Γ“Μ÷÷ΤχΧεΘ§»τΫΪ≤ζ…ζΒΡΤχΧεΆ®»κ Δ”–Ήœ…Ϊ ·»ο»ή“ΚΒΡ ‘Ιή÷–Θ§ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΔέ_________ΘΜΫΪ¥Υ ‘Ιή‘ΎΨΤΨΪΒΤΜπ―φ…œΦ”»» ±Θ§“ΣΔή__________Θ®Χν Β―ι≤ΌΉςΘ©Θ§¥Υ ±Ιέ≤λΒΫΒΡœ÷œσ «Δί__________ΓΘ
Θ®2Θ©»τ”Ο»γΆΦ Β―ιΉΑ÷Ο÷Τ»Γ―θΤχΘ§Ρή”Ο≈≈Υ°Ζ® ’Φ·¥ΥΤχΧεΒΡ‘≠“ρ «ΘΚΥφΉ≈Ζ¥”ΠΫχ––Θ§Φ·ΤχΤΩΡΎΒΡΤχΧε‘ωΕύΘ§”…”Ύ―θΤχ≤Μ“Ή»ή”ΎΥ°“≤≤Μ”κΥ°ΖΔ…ζΖ¥”ΠΘ§ ΙΤΩΡΎ__________ΆβΫγ¥σΤχ―ΙΘ§‘Ύ―Ι«Ω≤νΒΡΉς”Οœ¬Θ§ΤΩΡΎ“ΚΧε±Μ―ΙΫχΥ°≤έΓΘ
ΓΨ¥πΑΗΓΩ≥ΛΨ±¬©ΕΖ ΔΌΔΎΔΏ CO2+H2O=H2CO3 ≤Μ ±ΒΊ“ΤΕ· ‘ΙήΘ®Μρœ» Ι ‘ΙήΒΉ≤ΩΨυ‘» ή»»Θ§»ΜΚσ”ΟΨΤΨΪΒΤΆβ―φΙΧΕ®Φ”»»Θ© Κλ…Ϊ»ή“Κ±δΈΣΉœ…Ϊ ―Ι«Ω¥σ”Ύ
ΓΨΫβΈωΓΩ
Θ®1Θ©ΔΌ»τ≤Ι≥δ“Μ÷÷“«Τς≥ΛΨ±¬©ΕΖ≤Δάϊ”Ο…œ ω“«ΤςΘΚΔΌΔΎΔΏΚΆ≤ΩΖ÷“©ΤΖΩ…÷Τ»ΓΕΰ―θΜ·ΧΦΘΜΔέ»τΫΪ≤ζ…ζΒΡΕΰ―θΜ·ΧΦΆ®»κ Δ”–Ήœ…Ϊ ·»ο»ή“ΚΒΡ ‘Ιή÷–Θ§Εΰ―θΜ·ΧΦΚΆΥ°Ζ¥”Π…ζ≥…ΧΦΥαΘ§Μ·―ßΖΫ≥Χ ΫΈΣΘΚH2O+CO2=H2CO3ΘΜΫΪ¥Υ ‘Ιή‘ΎΨΤΨΪΒΤΜπ―φ…œΦ”»» ±Θ§“Σ≤Μ ±ΒΊ“ΤΕ· ‘ΙήΘ®Μρœ» Ι ‘ΙήΒΉ≤ΩΨυ‘» ή»»Θ§»ΜΚσ”ΟΨΤΨΪΒΤΆβ―φΙΧΕ®Φ”»»Θ©ΘΜΦ”»»ΚσΧΦΥαΖ÷Ϋβ≥…Εΰ―θΜ·ΧΦΚΆΥ°Θ§Ιέ≤λΒΫΒΡœ÷œσ «ΘΚ»ή“Κ”…Κλ…Ϊ±δ≥…Ήœ…ΪΘΜ
Θ®2Θ©»τ”Ο»γΆΦ Β―ιΉΑ÷Ο÷Τ»Γ―θΤχΘ§Ρή”Ο≈≈Υ°Ζ® ’Φ·¥ΥΤχΧεΒΡ‘≠“ρ «ΘΚΥφΉ≈Ζ¥”ΠΫχ––Θ§Φ·ΤχΤΩΡΎΒΡΤχΧε‘ωΕύΘ§”…”Ύ―θΤχ≤Μ“Ή»ή”ΎΥ°“≤≤Μ”κΥ°ΖΔ…ζΖ¥”ΠΘ§ ΙΤΩΡΎ―Ι«Ω¥σ”ΎΆβΫγ¥σΤχ―ΙΘ§‘Ύ―Ι«Ω≤νΒΡΉς”Οœ¬Θ§ΤΩΡΎ“ΚΧε±Μ―ΙΫχΥ°≤έΓΘ

 –¬ΩΈ±ξΆ§≤Ϋ―ΒΝΖœΒΝ–¥πΑΗ
–¬ΩΈ±ξΆ§≤Ϋ―ΒΝΖœΒΝ–¥πΑΗ “ΜœΏΟϊ ΠΩΎΥψ”Π”ΟΧβΧλΧλΝΖ“Μ±Ψ»ΪœΒΝ–¥πΑΗ
“ΜœΏΟϊ ΠΩΎΥψ”Π”ΟΧβΧλΧλΝΖ“Μ±Ψ»ΪœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ–Γ”ΔΆ§―ßΈΣΝΥ≤βΕ®Ρ≥ΒΊ«χ ·Μ“ ·―υΤΖ÷–ΧΦΥαΗΤΒΡ÷ ΝΩΖ÷ ΐΘ§»ΓΗΟ―υΤΖ15gΘ§œ÷ΫΪ75mLΒΡœΓ―ΈΥαΖ÷»ΐ¥ΈΦ”»κ ·Μ“ ·―υΤΖ÷–Θ§ΟΩ¥Έ≥δΖ÷Ζ¥”ΠΚσ≤βΒΟ…ζ≥…ΤχΧεΒΡ÷ ΝΩΘ§ Β―ι ΐΨί»γœ¬±μΘΚ
Β―ι | ΒΎ“Μ¥Έ | ΒΎΕΰ¥Έ | ΒΎ»ΐ¥Έ |
Φ”»κœΓ―ΈΥαΒΡΝΩ/mL | 25 | 25 | 25 |
…ζ≥…ΤχΧεΒΡ÷ ΝΩ/g | 2.2 | m | 1.1 |
‘«σΘΚ
Θ®1Θ©mΒΡ÷Β «ΓΓ ΓΓgΘ°
Θ®2Θ©«σΗΟ―υΤΖ÷–ΧΦΥαΗΤΒΡ÷ ΝΩΖ÷ ΐΘ®±ΘΝτ“ΜΈΜ–Γ ΐΘ©Θ°
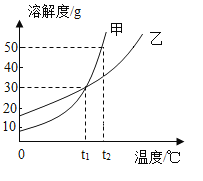
ΓΨΧβΡΩΓΩœ¬Ν–ΥΡΗωΆΦœώΖ¥”≥ΝΥΕ‘”Π Β―ιΙΐ≥Χ÷–œύΙΊΝΩΒΡ±δΜ·Θ§Τδ÷–¥μΈσΒΡ «
|
|
|
|
AΘ°≤ΩΖ÷±δ÷ ΒΡNaOH»ή“Κ÷–ΒΈΦ”œΓ―ΈΥα | BΘ°HClΤχΧεΒΡ»ήΫβΕ» ήΈ¬Ε»”ΑœλΒΡ±δΜ·«ζœΏ | CΘ°Β»÷ ΝΩΒΡMgΖέΚΆFeΖέ”κΉψΝΩœύΆ§≈®Ε»ΒΡœΓ―ΈΥαΖ¥”Π | DΘ°ΝρΥα»ή“ΚœΓ ΆΙΐ≥Χ÷–pHΒΡ±δΜ·«ζœΏ |
A. A B. B C. C D. D
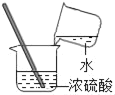
ΓΨΧβΡΩΓΩ–ΓΈΡΆ§―ß»Γ»πΫπ – ·Μ“ ·―υΤΖ20gΫχ––≤βΕ® Β―ιΘ§œ÷ΫΪ100gœΓ―ΈΥαΖ÷Έε¥ΈΦ”»κ ·Μ“ ·―υΤΖ÷–(‘”÷ ≤Μ»ή”ΎΥ°“≤≤Μ≤Έ”κΖ¥”Π)Θ§≥δΖ÷Ζ¥”ΠΚσ≤βΒΟ…ζ≥…ΤχΧεΒΡΉή÷ ΝΩ»γ±μΥυ ΨΓΘ
ΒΎ1¥Έ | ΒΎ2¥Έ | ΒΎ3¥Έ | ΒΎ4¥Έ | ΒΎ5¥Έ | |
Φ”»κœΓ―ΈΥαΒΡ÷ ΝΩ/g | 20 | 20 | 20 | 20 | 20 |
…ζ≥…ΤχΧεΒΡΉή÷ ΝΩ/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
‘«σ:
Θ®1Θ©mΒΡ÷ΒΈΣ_______ΘΜ
Θ®2Θ©ΒΎ______¥ΈΈΣ«ΓΚΟΆξ»ΪΖ¥”ΠΘΜ
Θ®3Θ©ΦΤΥψΗΟ ·Μ“ ·―υΤΖ÷–ΧΦΥαΗΤΒΡ÷ ΝΩΖ÷ ΐΓΘ(–¥≥ωΦΤΥψΙΐ≥Χ)______
ΓΨΧβΡΩΓΩœ¬ΆΦ « Β―ι “÷Τ±ΗΤχΧεΒΡ≤ΩΖ÷ΉΑ÷ΟΓΘ
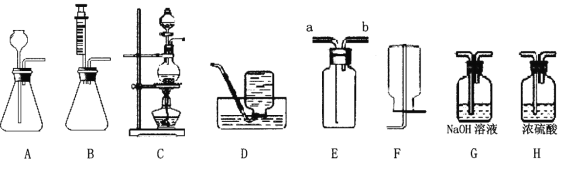
Θ®1Θ©…œΆΦΉΑ÷ΟΩ…“‘÷Τ»Γ≤ΜΆ§ΤχΧε,«κΆξ≥…œ¬±μΩ’ΗώΓΘ
Ζ¥”ΠΈοΚΆΖ¥”ΠΧθΦΰ | ÷Τ»ΓΒΡΤχΧε | ΖΔ…ζΉΑ÷ΟΘ®ΧνΉ÷ΡΗΘ© | ’Φ·ΉΑ÷ΟΘ®ΧνΉ÷ΡΗΘ© |
H2O2»ή“ΚΚΆMnO2ΙΧΧε,≥ΘΈ¬ | ______ | AΜρB | ΜρE |
―«ΝρΥαΡΤΘ®Na2SO3Θ©ΙΧΧεΚΆ≈®ΝρΥα,≥ΘΈ¬ | SO2 | ______ | E |
ΦΉΥαΘ® HCOOHΘ©ΚΆ≈®ΝρΥα,Φ”»» | CO | ______ | D |
Θ®2Θ©”ΟAΉΑ÷Ο÷Τ»ΓO2 ±,Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________________,»τ“ΣΒΟΒΫΗ…‘οΒΡO2,Υυ―Γ‘ώΉΑ÷ΟΒΡΝ§Ϋ”Υ≥–ρΈΣ:AΓζ____Γζ _____ Θ®ΧνΉ÷ΡΗ–ρΚ≈Θ©ΓΘ
Θ®3Θ©”ΟEΉΑ÷Ο ’Φ·SO2 ±,ΤχΧε”Π”…Θ®ΧνΓΑaΓ±ΜρΓΑbΓ±Θ©________ΕΥΫχ»κΓΘ