��Ŀ����
����Ŀ���������ƣ�Na2O2����һ�ֹ���������ˮ��Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2H2O�T4NaOH+O2����������Ϊ19��6g�IJ�����Na2O2��ֻ����NaOH���ʣ����뵽87��6gˮ�У�ǡ����ȫ��Ӧ��������Һ������Ϊ104g��ˮ�Ļӷ����Բ��ƣ�������㣺
��1����������������Ϊ__g��
��2����Ӧ��������Һ�����ʵ�����____g��
��3����������Һϡ�ͳ����ʵ���������Ϊ10%����Һ�����ˮ������_____g��
���𰸡�3.2g 20g 96g
��������
��1�����������غ㶨�ɣ���Ӧ����ٵ�����Ϊ����������������Ϊ��19.6g+87.6g-104g=3.2g��
��2����μӷ�Ӧ�Ĺ������Ƶ�����Ϊx�������������Ƶ�����Ϊy����
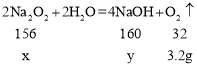
![]()
x=15.6g��y=16g
��Һ�����ʵ�����Ϊ��19.6g-15.6g+16g=20g��
�𣺷�Ӧ��������Һ�����ʵ�����Ϊ20g��
��3������Ҫ����ˮ������Ϊz��������Һϡ�ͣ������������䣬��
![]()
z=96g
����Ҫ����ˮ������Ϊ96g��

����Ŀ����ȥ���������е����ʣ���ѡ�õķ�������ȷ���ǣ�������
���ʣ�������Ϊ���ʣ� | �������� | |
A | CO2��CO�� | ͨ��������ȼ |
B | NaCl��Һ��Na2CO3�� | ��ϡ������ǡ�ò��ٲ�������Ϊֹ |
C | CaO��CaCO3�� | ���� |
D | ���ۣ�п�ۣ� | �ӹ���FeSO4��Һ��ַ�Ӧ����ˡ�ϴ�ӡ����� |
A. AB. BC. CD. D
����Ŀ��������ϵ��й����������ϵ��Ȳ��ó����ٴγ�Ϊ����ʩչ���յ���̨���������ʦ�и���������:����ʱ���ּ��Ͼ��ּӴף���ʹ�˱�������ɿڣ�ԭ���Ǵ��е��������Ͼ��е��Ҵ����������������±����Ǽ��ֳ���������������������⣺
�������� | ������� | �������� | ������� | �������� |
��ѧʽ | C2H4O2 | C3H6O2 | C3H6O2 | X |
(1)�������(C2H4O2)��̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ _______��
(2)��������(C3H6O2)��̼Ԫ�ص���������Ϊ_______(��������ȷ��0.1%)��
(3)�ȽϹ�����ѧϰ��ѧ����Ҫ�������ݱ��Ʋ�X�Ļ�ѧʽΪ________��





