��Ŀ����
����Ŀ��ͨ����ѧѧϰ��������ʶ�ˡ��ᡱ�͡������ش��������⣺
��1���������������ᡢ����ȣ������ǵ�ˮ��Һ�����ڴ�����ͬ��������ţ�����ˣ������кܶ����ƵĻ�ѧ���ʣ��磺�� �ȣ�д�������ɣ���
��2�������������ơ��������Ƶȣ��������ƿ���ijЩ����ĸ�����������ܸ��������̼������ �� �������ƿ�����ʯ����ˮ��Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽΪ
��3����ͼ��ܷ����кͷ�Ӧ�������ճ������ũҵ�������й㷺��Ӧ�ã������᳧����ˮ�к�����������ʣ�������ʯ�ҽ��д�������Ӧ�Ļ�ѧ����ʽΪ ��
��4��2010��4��14���ຣ����������ֺ�������Ϊ����������Ⱥ�ڵ�����ˮ��ȫ���������߲���Ҫ����������ˮԴ���м�⣬��ÿɿ��Ŀ�ѧ���ݣ�������һƿˮ����Ҫ��ȡ�������ȣ�Ӧ��β����� ��
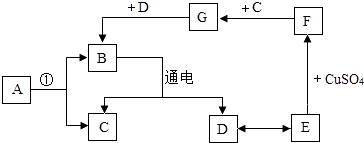
��5��ij��ѧ��ȤС�龭��һ��ʱ��ĵ�����̽����ģ���������ԭ�����������ͼ��ʾװ�ã�һ���ų�������ķ�ˮ����һ���ų����������Ƶķ�ˮ��Ȼ���ڳ��л�ϣ�����ַ�Ӧ���ŷţ����ù���һ���ų�����������10%�ķ�Һ400�֣��躬����9.8%�ķ�ˮ���ٶֲ�����ȫ�кͣ� 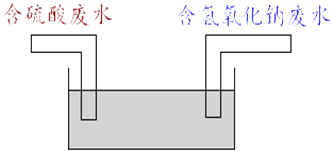
���𰸡�
��1��H+����ʹ��ɫʯ����Һ��죬ʹ��ɫ��̪��Һ����ɫ���������кͷ�Ӧ
��2�������������������̼��Ӧ��CaO+H2O=Ca��OH��2
��3��H2SO4+Ca��OH��2=CaSO4��+2H2O
��4��ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ
��5���⣺���躬����9.8%�ķ�ˮ������Ϊx
H2SO4+ | 2NaOH�TNa2SO4+2H2O |
98 | 80 |
x98% | 400t��10% |
![]()
x=50t
���躬����9.8%�ķ�ˮ������Ϊ50t
���������⣺��1������Ϊ������ˮ��Һ�е������������ȫ���������ӵĻ������������ˮ��Һ�о�����ͬ��H+ �� ����H+���ڻ������ͨ�Կ�֪����ʹ��ɫʯ����Һ��죬ʹ��ɫ��̪��Һ����ɫ���������кͷ�Ӧ������ϻ��ý�����Ӧ���ܺͽ��������ﷴӦ���ܺͲ����η�Ӧ�ȣ���2���������Ƶ����ڼ���������������Ե������������̼�Ͷ�������ȣ���Ϊ������֮��Ӧ���������ƿ�����ʯ����ˮ��Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O=Ca��OH��2�� ��������������������̼��Ӧ��CaO+H2O=Ca��OH��2����3����ʯ�ҳɷ֣�Ca��OH��2�����ڼH2SO4���ᣬ����кͷ�Ӧ�����Σ�CaSO4����ˮ��H2O������ȷд����Ӧ�������Ļ�ѧʽ���ٽ�����ʽ��ƽ���ɣ�
�ʷ�Ӧ����ʽΪ��H2SO4+Ca��OH��2=CaSO4��+2H2O����4��ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
���ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
�����㾫����������Ҫ�������кͷ�Ӧ����Ӧ�ú���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����֪ʶ�㣬��Ҫ�����кͷ�Ӧ����������������κ�ˮ�ķ�Ӧ��ע�⣺a����ƽ b������ c�����Ų�����ȷ�����⣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�