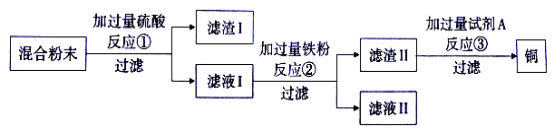
��Ŀ����
����Ŀ�������ÿ��ˮ�ܽ���������������0.003gʱ����ͻ���������������(CaO2)��һ�ֻ�ѧ����������Ӧ�Ļ�ѧ����ʽΪ�� 2CaO2+aH2O===2Ca(OH) 2+O2�����ֽ�2.88g�������Ʒ���ʢ��200Lˮ������С���ش�
(1)��ѧ����ʽ�л�ѧ������a����ֵΪ ��
(2)ͨ�������������ַ�Ӧ�������ˮ���������Ƿ�������Ҫ��(���������������ȫ����ˮ���������غ��Բ���)��
���𰸡�(1)2��(2)�����ˮ���������������Ҫ��
����������1�����������غ㶨�ɣ���Ӧǰ��ԭ�ӵ��������Ŀ���䣬Hԭ�ӵ���Ŀ2a=2��2��a=2��
��2��������O2������Ϊx��
2CaO2+2H2O�T2Ca��OH��2+O2����
144 32
2.88g x
![]()
x= 0.64g��
200Lˮ�����������ܽ�����������Ϊ��200L��0.003g/L=0.6g��
��Ϊ��Ӧ���ɵ��������������������������˷������Ҫ����

��ϰ��ϵ�д�
�����Ŀ