��Ŀ����
ij��ѧ�С�����ô�����ȡ�ռ��������ʵ�飺
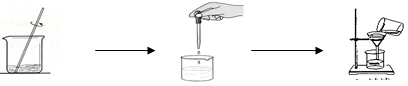
��ȡ������Ʒ���������ʲ�����ˮ��Ҳ�����������ʷ�Ӧ���������м���34.1gˮʹ����ȫ�ܽ⣬Ȼ���ټ���100g��������������Ϊ17.1%������������Һ����ǡ����ȫ��Ӧ�����˵õ�29.1g������һ�ֲ�������Һ����ش����⣺
��1������ʵ�鲽���У���ָ�������еĴ��� ;
��2����д��������Ӧ�Ļ�ѧ����ʽ ;
��3���г��μӷ�Ӧ��������������x���ı���ʽ ��
��4������Ʒ�Ĵ���Ϊ ��
��5����Ӧ��������Һ��������������Ϊ ��
��6���ֽ���Ӧ��������ҺŨ����10%����Һ�Ա��ã�Ũ��ʱû�о�����������������������ˮ������Ϊ ��

��ȡ������Ʒ���������ʲ�����ˮ��Ҳ�����������ʷ�Ӧ���������м���34.1gˮʹ����ȫ�ܽ⣬Ȼ���ټ���100g��������������Ϊ17.1%������������Һ����ǡ����ȫ��Ӧ�����˵õ�29.1g������һ�ֲ�������Һ����ش����⣺
��1������ʵ�鲽���У���ָ�������еĴ��� ;
��2����д��������Ӧ�Ļ�ѧ����ʽ ;
��3���г��μӷ�Ӧ��������������x���ı���ʽ ��
��4������Ʒ�Ĵ���Ϊ ��
��5����Ӧ��������Һ��������������Ϊ ��
��6���ֽ���Ӧ��������ҺŨ����10%����Һ�Ա��ã�Ũ��ʱû�о�����������������������ˮ������Ϊ ��
(1)����ʱû���ò�����������©�����û�н����ձ��ڱ�
(2)Ba��OH��2+Na2CO3=2NaOH+BaCO3��
(3)106/171=x/17.1g
(4)53%
(5)6.4%
��6��45g
(2)Ba��OH��2+Na2CO3=2NaOH+BaCO3��
(3)106/171=x/17.1g
(4)53%
(5)6.4%
��6��45g
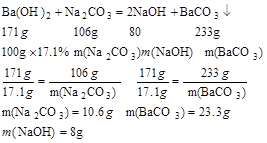
��Ʒ������10.6g+��29.1g-23.3g��=16.4g��
��Ӧ����Һ������34.1g+100g+16.4g-29.1g=121.4g
��2��Ba��OH��2+Na2CO3=2NaOH+BaCO3��
��3��

��4������Ʒ�Ĵ���Ϊ=
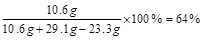
��5��

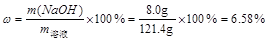

��ϰ��ϵ�д�
�����Ŀ
 �������йأ�һ���������Һ��
�������йأ�һ���������Һ��