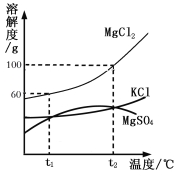
��Ŀ����
����Ŀ��������ͼ��ʾ��װ�ÿɽ���һ����̼��ԭ��������ʵ�飬���У�������������ʵ��ı������� ʢ������������һ֧����֧�ܵ��Թܣ���֧�ܿ����ڵ����Թ��ڵ����壻�ƾ��ƴ����֣�����װ�õ����������ã�ʵ��ǰ�����ɼ�a��b�����ڹر�״̬����ʼʵ��ʱ����a��ͨ�뵪��һ��ʱ����ȼ�ƾ��ƣ��ٹر�a����b������ϵ��ѧ֪ʶ�ش��������⣺
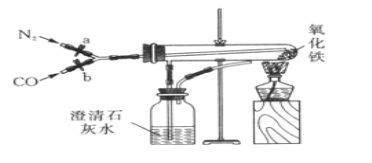
��1����ʵ������п��Թ۲쵽�������У�_________________��_________________��
��2���Թ���һ����̼����������Ӧ�Ļ�ѧ����ʽΪ____________________________��
��3��ʵ�����ʱ��Ӧ�ȴ�a����___________��Ȼ��___________������ͨ����ֱ��װ����ȴ����ѡ�A����B����A��Ϩ��ƾ��� B���ر�b
��4�����Թ���ȴ�����ɵĺ�ɫ��ĩ���ڰ�ֽ�Ϻ��ô���������ɫ��ĩ����ɫ��ĩȫ������������ȡ������ɫ��ĩ������������ͭ��Һ�У�������ֺ�ɫ��ĩ���ֱ�졣
��������⡿�������ĺ�ɫ��ĩ�ijɷ���ʲô�أ�
���������ϡ���������������ֻ���������Ǻ���ɫ������Ǻ�ɫ��ֻ�����������ڿ����л�ܿ챻��������������ֻ�������������д��ԡ�
���ۺ�̽�֡�ͬѧ�ǵó��˴������õ�����������___________________��
��5�����������ͬ�Ƕ����۸�ʵ����ŵ㣺_______________��_________________��
���𰸡���1������ɫ��ĩ��ڣ�����ʯ��ˮ����� ��2��3CO + Fe2O3 ![]() 2Fe + 3CO2
2Fe + 3CO2
��3��B��A ��4��Fe��Fe3O4 ��5����Լ��Դ����������ȫ��
��������
�����������1��һ����̼��ԭ���������ɵ������Ͷ�����̼���������к�ɫ��ĩ��ɺ�ɫ�������ʯ��ˮ����ǣ�
��2��һ����̼��ԭ���������ɵ������Ͷ�����̼��3CO + Fe2O3 ![]() 2Fe + 3CO2��
2Fe + 3CO2��
��3���÷�Ӧ��������ֹͣ���ȣ���ȴ����ֹͣͨһ����̼��Ŀ���Ƿ�ֹ�ѻ�ԭ�����ֱ�������
��4��������ɫ��ĩ������������ͭ��Һ�У�����������ͭ��Ӧ����ͭ����������������ֻ�в��ֺ�ɫ��ĩ��ɺ�ɫ�����ⲿ�ֺ�ɫ��ĩ���ܱ�����������������������ʣ���Ǻ�ɫ��ĩ���������������ʺ�ɫ��ĩ�����������������Ļ���
��5����ʵ���еĶ�����̼�����գ�δ��Ӧ��һ����̼Ҳû���ŷŵ������У������ռ������ˣ��ʸ�ʵ����ŵ�����Լ��Դ����������ȫ��