��Ŀ����
����Ŀ��ij����С���һ�������ˮ���ŷŵķ�Һ�к���CuSO4��H2SO4��
(1)CuSO4���ؽ����Σ����뵰���ʷ���______�仯(ѡ���������ѧ��)��ʹ������ʧȥԭ�����������ܣ�����CuSO4�ж���
(2)Ϊ�˲ⶨ��Һ��CuSO4��������������С��ȡ100g��Һ����μ���NaOH��Һ���������������Cu(OH)2����������������NaOH��Һ��������ϵ��ͼ��ʾ��
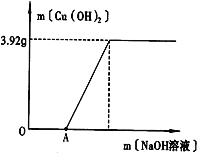
��ʵ����������Cu(OH)2������������_____g��ͼ��OA��δ����Cu(OH)2������ԭ��:______��
�ڼ����Һ��CuSO4����������(�����ȷ��0.1%)��_____����֪��CuSO4+2NaOH=Cu(OH)2��+Na2SO4��
������ȡl00g��Һ����������μ��������BaCl2��Һ��ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�������Ƶ�����Ϊmg����_____ (��ܡ����ܡ�)�ú�m�Ĵ���ʽ�����Һ��CuSO4������������
���𰸡���ѧ 3.92 ������ͭ������ˮ�������ܹ����ᷴӦ���ɿ��ܵ��Ȼ�ͭ������OA�����������ƺ����ᷴӦ������������ͭ��Ӧ 6.4% ����
��������
��1��CuSO4���ؽ����Σ����뵰���ʷ�����ѧ�仯��ʹ������ʧȥԭ�����������ܣ�����CuSO4�ж���
��2������ͼ��֪ʵ����������Cu��OH��2������������ 3.92 g��ͼ��OA��δ����Cu��OH��2������ԭ��������ͭ������ˮ�������ܹ����ᷴӦ���ɿ��ܵ��Ȼ�ͭ������OA�����������ƺ����ᷴӦ������������ͭ��Ӧ��
���裺��Һ��CuSO4����������Ϊx��
![]()
![]()
x��6.4%
���������������ͭ���ܺ��Ȼ�����Ӧ���ɳ��������Բ��������ᱵ�����������Һ��CuSO4������������