��Ŀ����
ijУ��ȤС��ͬѧΪ̽�����Ļ�ѧ���ʣ���ͼ��������������ʵ�飬��ش��й����⣺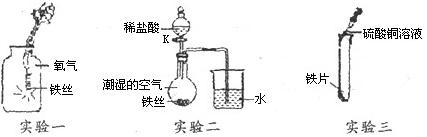
��1��ʵ��һ����������Ӧ�Ļ�ѧ����ʽ�� ��2�֣���
ʵ���������ܲ����IJ�������� ��
��2��ʵ������ر�Kһ��ʱ��۲쵽������Һ����������K���μ�ϡ���ᣬ�۲쵽������Һ���½������ܿ�������ð�����ر�K��������Һ��������ԭ���� ��������Һ���½���ԭ���� �����û�ѧ����ʽ���ͣ���2�֣�
��3��ʵ������ʵ��۲쵽�������ǣ� ��
��һ����˵�� ���������ͭ�����Ļ�Ը�ǿ��
��1��3Fe+2O2 Fe3O4��2�֣�������ƿ����
Fe3O4��2�֣�������ƿ����
��2��������������ƿ�ڵ�������ƿ����ѹС��������ѹ��Һ��������Fe+2HCl=FeCl2+H2��
��3���к�ɫ������������ɫ��Һ��dz��ɫ����
���������������1��ʵ��һ����˿ȼ�յ�ʵ�飬���Է�Ӧ�Ļ�ѧ����ʽ�ǣ�3Fe+2O2 Fe3O4��ʵ��ǰҪ��ƿ����ƿ�ײ���������ϸɳ���������ˮ����ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը��
Fe3O4��ʵ��ǰҪ��ƿ����ƿ�ײ���������ϸɳ���������ˮ����ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը��
��2�������п�����ˮ������£������⣬�ر�Kһ��ʱ���������������ƿ�ڵ�������ƿ����ѹС��������ѹ��Һ����������K���μ�ϡ���ᣬ������ϡ���ᷴӦ����������ʹ��ƿ�ڵ��������࣬ѹǿ�������Թ۲쵽������Һ���½��������ķ�Ӧ��ѧ����ʽ��Fe+2HCl=FeCl2+H2��
��3���������Ļ�Ա�ͭǿ�����������û�������ͭ��Һ�е�ͭ�����������ǣ��к�ɫ������������ɫ��Һ��dz��ɫ
���㣺����������������Ļ�ѧ����

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д���6��) 2011��9��29��21ʱ���칬һ�š�Ŀ��������ھ�Ȫ���Ƿ������ķ��䣬�����й����ռ�ʵ���ң����ֱ������۰˺š����۾źš�����ʮ�ŷɴ��Խӣ��Ӷ�������һ���й��ռ�ʵ���ҡ���ṹ��Ҫ�����ⲿ���塢ʵ��ա���Դ�ա�ȼ��ȼ��ϵͳ���Խӻ�����̫���ܵ��ȣ������������������µ�ʯī�ߡ�����֡��ѡ�����ͭ�ȵȡ��Իش��������⣺
��1�����������______________________��������������������
��2������ͭ�Ľ������________ �������Ľ�����ԣ�����ڡ����� С�ڡ��������ڡ���������Ƽ�ʵ����֤���û�ѧ����ʽ��ʾ��___________________
|
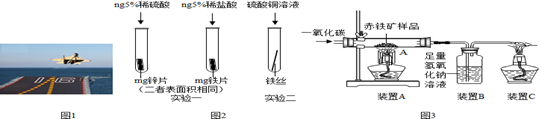
�Ϻ����й��Ĵ�������������Ȼ��Դ��Ϊ�ḻ�ĺ����Ϻ�������Դ�ḻ��������̫�������˵ġ��ʺ�Ҫ�������������������æ�ĺ��ߣ���������ܱ߹��ҵĿ��ӣ�
��1����ˮ���� ������������������ˮɹ�����ڻ�������������� ��������ţ�
| A������ | B������ | C������ | D������ |
��3���ڡ��Ϻ�һ�š����̳����������У�����������������ʶ�Ŀ���������ͭ��������ͭ�⣻�������⼣�߰ߣ��д���٣���˵����ͭ���������ֽ����Ļ˳����ǿ������˳���� ��
��4������������������������ ����������ϡ��ϳɲ��ϡ�����������ԭ��������һ����̼����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ ��
�ҹ������������Ӳ�Ҵ�1999�꿪ʼ���У�һԪ��Ϊ��о������Ni��,��DZ�Ϊ��о��ͭ�Ͻ�һ�DZ�Ϊ���Ͻ����֡�
��1����������Ӳ���õ��IJ��϶��� ����ϳɲ��ϡ��������ϡ�����
��2��ѡ������Ӳ�ҵIJ��ϲ���Ҫ���ǵ������� ������ţ���
| A�������ĵ����� | B����������ʴ�� |
| C��������Ӳ�� | D�������۸���Ӳ����ֵ���Ǻ϶� |
��1��������������������ͷ�չ����Ҫ���ʻ�����
��סլ����������Ľ������ϣ������������ڸ��ϲ��ϵ����� ������ĸ����
a��ˮ�� b��PVC��ˮ�ܵ� c������ d���ֽ������
���ִ������벻���������ϣ����и����ķ��������У�����Ч����ã�����Է���Ҳ�������� ��������ĸ����
a��Ϳ���� b�������ϲ㣨���ܣ� c���Ƴɲ����
�۷�Ʒ�չ���Ա���ֽ��������������ޡ������⡱����������������������Ϊ�������������ʻ��ã�����Ϊ�����ޡ������⡱ԭ������ ����������ɳ�ȥ�����ϵ����⣨��Ҫ�ɷ�ΪFe2O3��������д����ȥ����Ļ�ѧ����ʽ�� ����
�ܷϾɵ���к��й���������ⶪ�����������Ⱦ����в���ཡ��������Ԫ�ط������� �����������ڳ����µ�״̬���� �������õĸɵ���ڲ������Ȼ�狀͵����ʣ���ʵ���ҷ����Ȼ�狀Ͷ������̻����ɽ��еIJ����ǣ��ܽ⡢���˺��� �����õ����Ȼ����ũҵ���ֿ������� ����
��2��2012��3�£��¡������������������İ䲼�������ҶԻ�������Ľ�һ�����ӡ�
�������ϼ�װβ��������װ�ÿ���ʹNO��CO���Ӧת��Ϊ�����к��е��������壬���Ʒֱ�Ϊ
�� ������ ����
��úȼ�ղ�����SO2���γɵ������У�SO2����ת���ɵ������� ���ѧʽ������ú�м�������ʯ��ʯ���������Ĺ�ͬ�����£�������úȼ�ղ�����SO2��Ӧ��������ƺͶ�����̼���壬������Ӧ�Ļ�ѧ����ʽΪ�� ����
��3��ij��ʳƷ�����ϱ�ǩ��ͼ��ʾ��
| ���ϣ� С��� ���� ���� ����� ̼������ �������Ƶ� |
�ٸ������У����������ʵ��������� ����������֬���������� ��
�ڸ������е��� �����з������á�
����д��������۵�ʵ�鷽���������� ����
 ���ˣ����õ�����ϴ�ӡ�����������Ƶ�����Ϊa�ˣ�
���ˣ����õ�����ϴ�ӡ�����������Ƶ�����Ϊa�ˣ� ���ˣ����õ��Ĺ���ϴ�ӡ�����������Ƶ�����Ϊb�ˣ�
���ˣ����õ��Ĺ���ϴ�ӡ�����������Ƶ�����Ϊb�ˣ�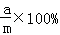 ��
��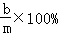 ��
��