��Ŀ����
 ˮ����Һ�������������������ʮ����Ҫ�����ã�����������ܽ�ȱ���
ˮ����Һ�������������������ʮ����Ҫ�����ã�����������ܽ�ȱ����ܽ�����ߣ��ش��������⣺
| �¶�/�� �ܽ��/g |
0 | 20 | 40 | 60 | 80 |
| KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 |
| NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
�����
�����
���ܽ�����ߣ���2��������л����������Ȼ��ƣ���Ҫ�õ�����������صķ�����
���½ᾧ����ȴ�ȱ�����Һ
���½ᾧ����ȴ�ȱ�����Һ
����3��60��ʱ���������ֱ�ʢ��50g NaCl��KNO3���ձ��У�������100g��ˮ������ܽ��Ϊ������Һ����
NaCl
NaCl
��Һ�����������ձ���ʣ�����ȫ���ܽ⣬��Ϊ��������Һ�����з�����ʵ�ֵ����ۢ�
�ۢ�
������ţ��������£��ڼ�������ʣ��ۼ�������ˮ���ܽ��£��ݼ������������ʵIJ�������Һ��
��4����������������Һ����100g������������Ϊ2%��ϡ��Һ���ܶȾ�����1g/mL��������ʱ����������
��Ͳ����ͷ�ιܡ��ձ���������
��Ͳ����ͷ�ιܡ��ձ���������
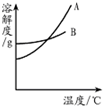
����������1�������ܽ�ȱ����ܽ�����߷���A���������ʵ��ܽ�����ߣ�
��2����������ء��Ȼ��Ƶ��ܽ�����¶ȱ仯����������ᴿ�ķ�����
��3������NaCl��KNO3��60��ʱ���ܽ�ȷ������㣻
��4������Ũ��Һ����ϡ��Һ���������������
��2����������ء��Ȼ��Ƶ��ܽ�����¶ȱ仯����������ᴿ�ķ�����
��3������NaCl��KNO3��60��ʱ���ܽ�ȷ������㣻
��4������Ũ��Һ����ϡ��Һ���������������
����⣺��1����A���ܽ�����߿�֪��A���ܽ�����¶ȵ����߱仯�ܴ�ϱ�����Է�������ص��ܽ�����¶ȵ����߶�����仯�ϴ�֪A������أ�
��2��������ء��Ȼ��Ƶ��ܽ�ȱ���֪������ص��ܽ�����¶ȵ����߱仯�ܴ��Ȼ��Ƶ��ܽ�����¶ȵ����߱仯�������ԣ���������л����������Ȼ��ƣ���Ҫ�õ�����������صķ����ǣ����½ᾧ������ȴ�ȱ�����Һ��
��3��������ء��Ȼ��Ƶ��ܽ�ȱ���֪����60��ʱ�ܽ�ȷֱ��ǣ�110g��37.3g�����ԣ�60��ʱ���������ֱ�ʢ��50g NaCl��KNO3���ձ��У�������100g��ˮ������ܽ��Ϊ������Һ����NaCl��Һ���������ձ���ʣ�����ȫ���ܽ⣬��Ϊ��������Һ����ʵ�ֵ��Ǽ�������ˮ��������������ʵIJ�������Һ���Ȼ��Ƶ��ܽ�����¶�Ӱ�첻�����¡����²����У�����������Ȼ�DZ�����Һ���������У�
��4����������������Һ����100g������������Ϊ2%��ϡ��Һ���ܶȾ�����1g/mL�����ɾ����ʵ�����Դ�ڱ�����Һ�������v=
��������Ҫ������Һ�������ˮ�������ʹ����Ͳ��ȡ��ͬʱ��Ҫ��ͷ�ιܶ��ݣ���Һ�����ձ�����ˮ��ϣ�����������ʹ֮���ȣ�
�ʴ�Ϊ����1������أ���2�����½ᾧ������ȴ�ȱ�����Һ����3���ۢݣ���4����Ͳ����ͷ�ιܡ��ձ�����������
��2��������ء��Ȼ��Ƶ��ܽ�ȱ���֪������ص��ܽ�����¶ȵ����߱仯�ܴ��Ȼ��Ƶ��ܽ�����¶ȵ����߱仯�������ԣ���������л����������Ȼ��ƣ���Ҫ�õ�����������صķ����ǣ����½ᾧ������ȴ�ȱ�����Һ��
��3��������ء��Ȼ��Ƶ��ܽ�ȱ���֪����60��ʱ�ܽ�ȷֱ��ǣ�110g��37.3g�����ԣ�60��ʱ���������ֱ�ʢ��50g NaCl��KNO3���ձ��У�������100g��ˮ������ܽ��Ϊ������Һ����NaCl��Һ���������ձ���ʣ�����ȫ���ܽ⣬��Ϊ��������Һ����ʵ�ֵ��Ǽ�������ˮ��������������ʵIJ�������Һ���Ȼ��Ƶ��ܽ�����¶�Ӱ�첻�����¡����²����У�����������Ȼ�DZ�����Һ���������У�
��4����������������Һ����100g������������Ϊ2%��ϡ��Һ���ܶȾ�����1g/mL�����ɾ����ʵ�����Դ�ڱ�����Һ�������v=
| m |
| �� |
�ʴ�Ϊ����1������أ���2�����½ᾧ������ȴ�ȱ�����Һ����3���ۢݣ���4����Ͳ����ͷ�ιܡ��ձ�����������
�����������ܽ�������ܶ����ر�ʾ���ܽ�ȱ仯�Ĺ��ɣ��˽ⱥ����Һ�벻������Һ���ת�䷽����Ũ��Һ����ϡ��Һ���������ǽ����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��6�֣�ˮ����Һ�������������������������ʮ����Ҫ�����á�
��1����ˮ���о���ʹ�û���̿����Ҫ���û���̿��_______�ԡ�
��2������ͼ��ʵ��ֻ������������������ȷ��ˮ������Ԫ�غ���Ԫ����ɵģ��õ��˽��۵�������________��д����ʵ���з�����Ӧ�Ļ�ѧ����ʽ________��
��3���ձ���ʢ��һ���������¶�Ϊ80�桢����ΪM����Һ�������������»����У��ⶨ��ͬ�¶�ʱ��������M���������ⶨ�����¼���±���
| ��Һ���¶�/�� | 75 | 65 | 50 | 35 | 20 |
| ��������M������/g | 0 | 0 | 2.0 | 4.5 | 8.4 |
�� 65��ʱ������Һ�Ƿ�Ϊ������Һ�� ����ǡ����������жϡ�����
�ڽ�t��ʱ�ӽ����͵�M��Һ��ɱ�����Һ�����з�����һ���ܴﵽĿ�ĵ��� ������ĸ��ţ���
A. ���� B. ���� C. ������M
D. ��ˮ E. ����һ�ֹ���N F. ��������ˮ
G. ��t��ʱM�ı�����Һ���
�� 20��ʱ���ù����ĩM��ˮ����100g������������Ϊ5%��M��Һ�������õ��������У�������ƽ�������룩���ձ��� ��
 ��2013?ͨ����һģ��ˮ����Һ�������������������ʮ����Ҫ�����ã�����������ܽ�ȱ����ܽ�����ߣ�ͼ1�����ش��������⣺
��2013?ͨ����һģ��ˮ����Һ�������������������ʮ����Ҫ�����ã�����������ܽ�ȱ����ܽ�����ߣ�ͼ1�����ش��������⣺

 ��2012?��ƽ����ģ��ˮ����Һ�������������������������ʮ����Ҫ�����ã�
��2012?��ƽ����ģ��ˮ����Һ�������������������������ʮ����Ҫ�����ã�