��Ŀ����
����Ŀ�������̲��ŷḻ����Դ���밴Ҫ����ա�
��1������Ϊ�����ṩ��Ӫ���ḻ��ʳ�ġ���Ϻ������_____�ǻ�����������������֯����Ҫԭ�ϣ������и����ĵ�Ԫ�ؿ���Ԥ��_____��
��2���ҹ��ڿ�ȼ�����ɼ�������ȡ���ش�ͻ�ơ���ȼ����Ҫ���м���ˮ�����������CO2������������ʱ�������й¶�������У��ᵼ��_____�Ӿ磬ʹȫ�������ů������ȼ�յĻ�ѧ����ʽΪ_____��
��3����ˮͨ��������е������ɻ���Һˮ��Դ�ѷ����⡣
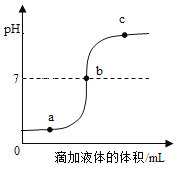
��ˮ���ˮ�����Ĺ����У������仯����_____������ĸ��ţ���
A ���Ӹ��� B �������� C ���Ӽ�� D ��������
�ڵ������ˮͨ������_____����������Ӳˮ������ˮ��
��4����ˮ̼���棬���Ի��������CO2��������⣬����ˮ��pHҲ����С���������ữ����ԭ����û�ѧ����ʽ��ʾΪ_____��
���𰸡������� ��״���״� ����ЧӦ CH4+2O2![]() CO2+2H2O C ����ˮ CO2+H2O�TH2CO3����
CO2+2H2O C ����ˮ CO2+H2O�TH2CO3����
��������
��1����Ϻ�����ĵ������ǻ�����������������֯����Ҫԭ�ϣ����������ĵ�Ԫ�ؿ���Ԥ����״���״�������ʣ���״���״�
��2��������̼����ᵼ������ЧӦ�Ӿ磬����ˮ�����������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ���������ЧӦ��CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
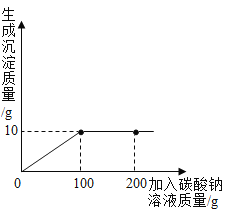
��3����ˮ���ˮ�����Ĺ����У��������仯���Ƿ��Ӹ����������������������࣬�����ı���Ƿ��Ӽ�������C��
�ڼ���ˮ��Ӳˮ������ˮ�����õ������Ƿ���ˮ��������ʹӲˮ�����ij��÷�������У��������ˮ��
��4��������̼���ں�ˮ�У���ˮ��Ӧ����̼�ᣬ���Ժ�ˮ��pH�����С��������ǿ����Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O�TH2CO3��
���CO2+H2O�TH2CO3��

����Ŀ����Ũ����Ĵ������£�������ᣨH2C2O4�����ȷֽ����������������ˮ��ij��ѧ������ȤС����������е������������������������ʵ��̽����
������⣺���ɵ�������������ʲô��
���룺
����1 | ����2 | ����3 | ����4 | ���� |
ֻ��CO | ֻ��CO2 | ֻ��SO2 | ����CO��CO2 | ���� |
��1�����������У�����_____���Դ��������ǣ�_____��

���ʵ�飺���ڲ���4������CO��CO2�����ʣ�ͬѧ�����������ʵ�飺
��2���۲쵽_____װ�ã�����ĸ���еij���ʯ��ˮ����ǣ�֤������ֽ���CO2���ɡ�
��3��������ʵ�������ֱܷ�֤������ֽ�������к���CO����Cװ���г���ʯ��ˮ������ǣ�Fװ����_____����Eװ���г���_____������
��4��ʵ����ۣ�ͨ��ʵ��̽��֤��������4����������ֽ�Ļ�ѧ����ʽ��_____��
��5���������ۣ���Cװ�õ�������_____��
����ʵ��װ�ô���һ�����Բ���֮������ĸĽ�������_____��