��Ŀ����
һͬѧ��ij�ִ��ν����ᴿʵ�飬�������ͼ��
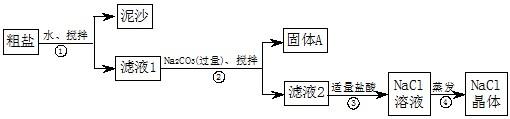
��ش�
��1������ٺ͢ڵIJ��������� ��
��2��������жϼ������ᡰ�������ķ����� ������ܼ�������ʱҪ�ò������Ͻ��裬����Ϊ�˷�ֹ �������������н϶����������ʱ��Ӧ ��������ʹˮ�����ɡ�
��3���������֤��
| �� �� | �� ֤ �� �� �� | �� �� | �� �� |
| �����:����A�к�CaCO3��MgCO3�� | ȡ��������A���Թ��У��μ�ϡ���ᣬ����Ϳ�г���ʯ��ˮ��С�ձ������Թܿڡ� |
| �������� |
| �����:����A�к�BaCO3�� | ȡ��������A���Թ��У��ȵ��� ���ٵ���Na2SO4��Һ | �����ݷų����ް�ɫ���� | |
| �����:����Ƶõ�NaCl�����л�����Na2SO4�� | ȡ����NaCl���������Թ��е�����ˮ��
| �������� |
��1�����ˣ���1�֣�
��2���μ������������ݷų�Ϊֹ��2�֣� �ֲ����ȣ�1�֣�����ɹ��壨����Һ���ɽ���1�֣� �� ֹͣ���ȣ���Ϩ��ƾ��ƣ�1�֣�
��3������������ݷų����ձ��ױ���ǣ���ʯ��ˮ����ǻ�ʯ��ˮ�а�ɫ������2�֣�
�������ϡ���ᣨ��ϡ���ᣩ��1�֣� ���������1�֣�
�������BaCl2[��Ba(OH)2����Ba(NO)3]��Һ��ϡHNO3��2�֣� �а�ɫ�����Ҳ�����ϡHNO3��2�֣�

 ��У����ϵ�д�
��У����ϵ�д�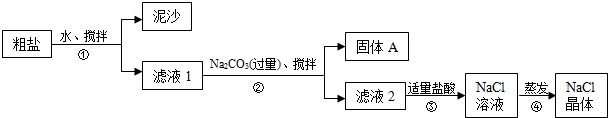
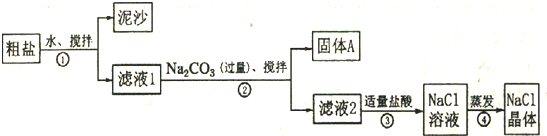
��ش�
��1������ٺ͢ڵIJ���������
��2���������Ϊ�õ������Ĺ���A���轫����A��ˮϴ�Ӹɾ��������ʵ��������A�Ƿ�ϴ��
��3���������֤��
| ���� | ��֤���� | ʵ������ | ���� |
| ����I������A�к���CaCO3 | ȡ��������A���Թ��У��μ�ϡ���ᣬ����Ϳ�г���ʯ��ˮ��С�ձ������Թܿ� | ___________ | ����I���� |
| ����II������A���BaCO3 |
ȡ��������A���Թ��У��ȵ���_________���ٵ���ϡNa2SO4��Һ | �����ݷų����ް�ɫ���� | ________ |
| ����III������Ƶõ�NaCl�����л�����Na2SO4 |
ȡ����NaCl�������Թ��У�������������ˮ�ܽ⣬��_______ | ________ | ����III���� |