��Ŀ����
����Ŀ����ʽ̼��ͭ����������ľ�ķ������ȡ���ҵ�ϳ��ں�������ͭ�ķ�ˮ�м���̼������Һ���õ���ʽ̼��ͭ������
��1����Ʒ�����м���Na2CO3��Һʱ����ӦҺpH�Բ�Ʒ��SO42���ĺ����Լ���ӦҺ��Cu2������Ч�ʵ�Ӱ����ͼ��ʾ������ʱ��ҺpH��ÿ�����_________���ҡ�
��2��ʵ��̽��
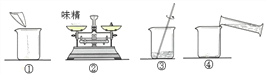
��.��֤��ʽ̼��ͭ�ķֽ�����ѡװ����ͼ��ʾ��

��ͬѧ��װ�ð�BCA��˳����Ͻ���ʵ�飬װ��A��ʢ�ŵ��Լ���������___________��װ��C�е�����Ϊ___________��װ��C��A˳���ܵߵ���ԭ����___________��
ʵ�������Bװ���з�ĩ��ȫ��ڡ����ں�ɫ���ʵijɷ֣�������Ϊ������̼��������ʵ��������ʺ�Ԫ���غ�Ƕ�˵���˲����������_____________��
��ͬѧΪȷ�ϸú�ɫ��ĩ������ͭ������̼�ۣ��������ʵ�飬������±���
ʵ�鲽�� | �۲쵽������ | ��ý��� | ��Ӧ�Ļ�ѧ����ʽ |
ȡ������ɫ��ĩ���Թ��У������м���������ϡ���ᣬ�� | _______ | ��ɫ��ĩ��Ϊ����ͭ | _______ |
��.��ͬѧ̽��CO��ԭCuO���ù������ijɷ֣�ʵ��װ������ͼ��

���ϣ�CO��ԭCuO��ʵ������У�������������Cu2O����ɫ���壩��Cu��
��Ӧǰ | ��Ӧ�� |
�����ܺ���ʢ����ͭ ��ĩ��������Ϊ62.0 g | �����ܺ���ʢ��ɫ ��ĩ��������Ϊ61.7 g |
�����ܵ�����Ϊ60.0 g | |
��1����K������ͨ��CO���壬һ��ʱ�����ȡ�����ɫ����ȫ�������ֹͣ���ȣ�����ͨ��CO����ֱ����������ȴ��
����ʵ�����ݼ����֪��CuO��ͭԪ�ص�����Ϊ_________g����ɫ�����ĩ�ɷ�Ϊ_________��
�������йظ�ʵ���˵����ȷ����_____________��
A����ͨ��һ��CO�ټ�����Ϊ���ž�װ���еĿ���
B��ʵ����������Ƚ������������߿��ڵ�װ�÷ֿ�����ֹ��Һ����
C��װ��ͼ�����߿��ڵ�װ�ü������ն�����̼�������ռ�һ����̼
D�����뵽�ձ��е���Һ�����Լ��ԣ�Ҳ����������
��.��ҵ�����Ƶõļ�ʽ̼��ͭ����϶࣬����ɱ�ʾΪ��xCuCO3��yCu(OH)2��zH2O��Ϊ�ⶨij�ֲ�Ʒ����ɣ���ͬѧ��Ƶ�ʵ�鷽���������²��裺�ٳ�ȡ12.0 g����Ʒ���ڸ��·ֽ⣻�۲������CO2������Ϊ2.2 g���ܲ������ˮ����������Ϊ1.8 g�������ʵ�����ݣ�����ȷ���ü�ʽ̼��ͭ�Ļ�ѧʽ��д��������̡�_______
���𰸡� 8.5 ���������̼���� ��ɫ�����Ϊ��ɫ A�г���ʯ��ˮ�е�ˮ��Բ�����ˮ�ļ����и��� ̼Ϊ��ɫ�ҷ�Ӧ������̼Ԫ�� ������ȫ���ܽ⣬��Һ��Ϊ��ɫ ����ʽ���� 1.6g Cu2O��Cu ABCD ��Ӧ�����ɵ�CuO������Ϊ12.0g- 2.2g -1.8g=8.0g
��x+y��CuO ~ x CO2 ~ (y+z) H2O
80(x+y ) 44x 18(y+z)
8.0g 2.2g 1.8g
80(x+y)/8.0g = 44x/ 2.2g = 18(y+z)/ 1.8g
x = y = z
��ʽ̼��ͭ�Ļ�ѧʽΪ��CuCO3��Cu(OH)2��H2O
��������������ѧ֪ʶ��������Ϣ֪����ҵ�ϳ��ں�������ͭ�ķ�ˮ�м���̼������Һ���õ���ʽ̼��ͭ��������1����Ʒ�����м���Na2CO3��Һʱ����ӦҺpH�Բ�Ʒ��SO42���ĺ����Լ���ӦҺ��Cu2������Ч�ʵ�Ӱ����ͼ��ʾ������ʱ��ҺpH��ÿ�����8.5 ���ҡ���2��ʵ��̽���â�.��֤��ʽ̼��ͭ�ķֽ�����ͬѧ��װ�ð�BCA��˳����Ͻ���ʵ�飬װ��A��ʢ�ŵ��Լ��������Ǽ��������̼���壻װ��C�е�����Ϊ��ɫ�����Ϊ��ɫ��װ��C��A˳���ܵߵ���ԭ����A�г���ʯ��ˮ�е�ˮ��Բ�����ˮ�ļ����и��š�ʵ�������Bװ���з�ĩ��ȫ��ڡ����ں�ɫ���ʵijɷ֣�������Ϊ������̼��������ʵ��������ʺ�Ԫ���غ�Ƕ�˵���˲����������̼Ϊ��ɫ�ҷ�Ӧ������̼Ԫ�ء���ͬѧΪȷ�ϸú�ɫ��ĩ������ͭ������̼�ۡ�
ʵ�鲽�� | �۲쵽������ | ��ý��� | ��Ӧ�Ļ�ѧ����ʽ |
ȡ������ɫ��ĩ���Թ��У������м���������ϡ���ᣬ�� | ����ȫ���ܽ⣬��Һ��Ϊ��ɫ�� | ��ɫ��ĩ��Ϊ����ͭ�� | CuO��H2SO4 ��H2O��CuSO4 |
��.��ͬѧ̽��CO��ԭCuO���ù������ijɷ֡����ϣ�CO��ԭCuO��ʵ������У�������������Cu2O����ɫ��������Cu����1����K������ͨ��CO���壬һ��ʱ�����ȡ�����ɫ����ȫ�������ֹͣ���ȣ�����ͨ��CO����ֱ����������ȴ�� ����ʵ�����ݼ����֪��CuO��ͭԪ�ص�����Ϊg����ɫ�����ĩ�ɷ�Ϊ�� CuO ![]() ��
��![]() ,x��1.6g����ɫ�����ĩ�ɷ�ΪCu2O��Cu ����A����ͨ��һ��CO�ټ�����Ϊ���ž�װ���еĿ�������A��ȷ��B��ʵ����������Ƚ������������߿��ڵ�װ�÷ֿ�����ֹ��Һ��������B��ȷ��C��װ��ͼ�����߿��ڵ�װ�ü������ն�����̼�������ռ�һ����̼����C��ȷ��D�����뵽�ձ��е���Һ�����Լ��ԣ�Ҳ���������ԡ���D��ȷ����.��ҵ�����Ƶõļ�ʽ̼��ͭ����϶࣬����ɱ�ʾΪ��xCuCO3��yCu(OH)2��zH2O����Ӧ�����ɵ�CuO������Ϊ12.0g- 2.2g -1.8g=8.0g��
,x��1.6g����ɫ�����ĩ�ɷ�ΪCu2O��Cu ����A����ͨ��һ��CO�ټ�����Ϊ���ž�װ���еĿ�������A��ȷ��B��ʵ����������Ƚ������������߿��ڵ�װ�÷ֿ�����ֹ��Һ��������B��ȷ��C��װ��ͼ�����߿��ڵ�װ�ü������ն�����̼�������ռ�һ����̼����C��ȷ��D�����뵽�ձ��е���Һ�����Լ��ԣ�Ҳ���������ԡ���D��ȷ����.��ҵ�����Ƶõļ�ʽ̼��ͭ����϶࣬����ɱ�ʾΪ��xCuCO3��yCu(OH)2��zH2O����Ӧ�����ɵ�CuO������Ϊ12.0g- 2.2g -1.8g=8.0g��
��x+y��CuO �� x CO2 ��(y+z) H2O
80(x+y ) 44x 18(y+z)
8.0g 2.2g 1.8g
80(x+y)/8.0g = 44x/ 2.2g = 18(y+z)/ 1.8g
x = y = z
��ʽ̼��ͭ�Ļ�ѧʽΪ��CuCO3��Cu(OH)2��H2O
�㾦�ñ�����Ҫ�����ʽ̼��ͭ���Ʊ����ֽ�����Լ�̽��CO��ԭCuO���ù������ijɷ֡�

����Ŀ�����г����Լ�ʹ����ȷ���� �� ����
ѡ�� | ���ʣ�������Ϊ���ʣ� | �����Լ� |
A | C��CuO�� | ���� |
B | CaCl2��Һ��HCl�� | Ba(OH)2��Һ |
C | CO��CO2�� | ��ʯ�ң�CaO��NaOH���� |
D | NaOH��Һ��Na2CO3�� | HCl��Һ |
A. A B. B C. C D. D