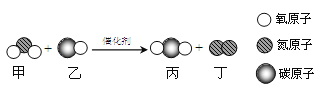
��Ŀ����
����Ŀ��ȼ�ϵĺ���ʹ���ǽ��������Ⱦ��Ҫ;����
����������Ϊ���������ȼ�ϡ������û�ѧ����ʽ����ԭ��________��
�ڽ����������Ƶ�����º���ϵͳ�����������࣬����Ϊ�����������˴�����________������ĸ��ţ���
a��������̼ b���������� c���������� d�������������
���ҹ��Ѿ��������ƹ�ʹ���Ҵ����ͣ����к��Ҵ���C2H5OH��10%���Ҵ���Ħ��������________��ÿĦ���Ҵ������к�________����ԭ�ӣ�
���Ҵ��ͼ��鶼�dz��õ�ȼ�ϣ���ȫȼ�պ������ͬ����ȼ��2mol�Ҵ��ͷų��Ķ�����̼��________g����ȼ���ͷų��Ķ�����̼��������ȣ�
���𰸡�2H2+O2![]() 2H2Obcd46g/mol6��6.02��1023����3.612��1024��64
2H2Obcd46g/mol6��6.02��1023����3.612��1024��64
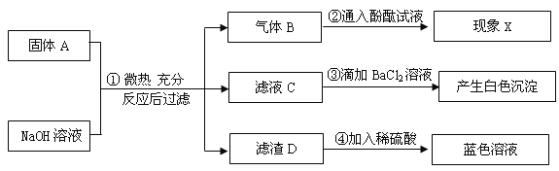
��������
��������ȼ�ղ�����ˮ�������ɷ֣�Ħ���ĺ�����ݻ�ѧ����ʽ����ķ������з������
������ȼ�ղ�����ˮ������Ⱦ������Ϊ������������Դ����Ӧ�Ļ�ѧ����ʽ��Ϊ��2H2+O2![]() 2H2O��
2H2O��
�����������������Լ��������������������������Ҫ����������������Ƶ�����º���ϵͳ�����������࣬����Ϊ�����������˴����ĵ�������������� ��������������ѡbcd��
���Ҵ�����Է���������46��Ħ��������46g/mol��ÿĦ���Ҵ��к���6��6.02��1023=3.612��1024����ԭ�ӣ�
����C2H5OH+3O2![]() 2CO2+3H2O ��֪��1Ħ���Ҵ���Ӧ����2Ħ��������̼����CH4+2O2
2CO2+3H2O ��֪��1Ħ���Ҵ���Ӧ����2Ħ��������̼����CH4+2O2![]() CO2+2H2O��֪��1Ħ�����鷴Ӧ����1Ħ��������̼����ȼ��2mol�Ҵ��ͷų��Ķ�����̼��ȼ��4mol����ų��Ķ�����̼������ȡ��������Է���������16��Ħ��������16g/mol����4mol����������ǣ�4mol��16g/mol=64g��
CO2+2H2O��֪��1Ħ�����鷴Ӧ����1Ħ��������̼����ȼ��2mol�Ҵ��ͷų��Ķ�����̼��ȼ��4mol����ų��Ķ�����̼������ȡ��������Է���������16��Ħ��������16g/mol����4mol����������ǣ�4mol��16g/mol=64g��

����Ŀ��(1)��ҵ���ü��ҷ�Ӧ�Ʊ�ȼ�ϱ���
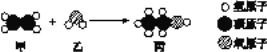
�ٱ���C��H��OԪ�ص�������Ϊ____________��
�ڸ÷�Ӧ�Ļ���������____________________��
(2)�Ƚ����л�ѧ���ﲢ��ɱ������ݡ�
H2O��H2O2��NH3 | ����֮���� |
��__________________________�� | |
��______ | |
| ��֮ͬ���� |
_____________________________�� | |
______________ |