��Ŀ����
��20g CaCl2��CaC03 �Ĺ���������뵽 65.9g ˮ�г���ܽ⣬������ 21.2%5Og ��Na2CO3��Һ��ǡ����ȫ��Ӧ��
��1��д���� Na2C03 ��Һ�����Ļ�ѧ��Ӧ����ʽ��
��2����ԭ���������� CaCl2 ��������
��3����������Һ��������
��4���������ɵ���Һ�������õ�t��ı�����Һ����ʱ���ܽ��Ϊ 36g/100gˮ���������������ٿ�ˮ��
�⣺��1���÷�Ӧ�ķ���ʽ��CaCl2 +Na2CO3 �TCaCO3��+2NaCl��
��2��������CaCO3������������x�������Ȼ��Ƶ�����Ϊy����������Ȼ��Ƶ�����Ϊz��
CaCl2 +Na2CO3 �TCaCO3��+2NaCl
111 106 100 117
z 50g��21.2% x y

x=10g y=11.7g z=11.1g
��3����������Һ��������11.1g+65.9g+50g-10g=117g��
��4������������ˮ��������w
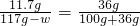
w=72.8g
�𣺣�1��CaCl2 +Na2CO3 �TCaCO3��+2NaCl����2��ԭ���������� CaCl2 ��������11.1g����3��������Һ��������117g��
��4���������ɵ���Һ�������õ�t��ı�����Һ����ʱ���ܽ��Ϊ 36g/100gˮ������������72.8g
��������1������̼���ƺ��Ȼ��Ʒ�Ӧ����̼��ƺ��Ȼ�����д��Ӧ�Ļ�ѧ����ʽ��
��2���ɲμӷ�Ӧ̼���Ƶ��������㷴Ӧ���Ȼ����������ɣ�
��3��������Һ����������ԭ���ǰ���ʵ�����������Һ�г���֮���ɣ�
��2�������ܽ�ȵ��йؼ���Ҫ���ɣ�
�����������ǶԻ�ѧ����ʽ�й�����Ŀ��飬����Ĺؼ�������Ӧ��Ҫ����й����ʵ��������ܽ��֪ʶ��Ӧ�ã�
��2��������CaCO3������������x�������Ȼ��Ƶ�����Ϊy����������Ȼ��Ƶ�����Ϊz��
CaCl2 +Na2CO3 �TCaCO3��+2NaCl
111 106 100 117
z 50g��21.2% x y

x=10g y=11.7g z=11.1g
��3����������Һ��������11.1g+65.9g+50g-10g=117g��
��4������������ˮ��������w
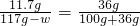
w=72.8g
�𣺣�1��CaCl2 +Na2CO3 �TCaCO3��+2NaCl����2��ԭ���������� CaCl2 ��������11.1g����3��������Һ��������117g��
��4���������ɵ���Һ�������õ�t��ı�����Һ����ʱ���ܽ��Ϊ 36g/100gˮ������������72.8g
��������1������̼���ƺ��Ȼ��Ʒ�Ӧ����̼��ƺ��Ȼ�����д��Ӧ�Ļ�ѧ����ʽ��
��2���ɲμӷ�Ӧ̼���Ƶ��������㷴Ӧ���Ȼ����������ɣ�
��3��������Һ����������ԭ���ǰ���ʵ�����������Һ�г���֮���ɣ�
��2�������ܽ�ȵ��йؼ���Ҫ���ɣ�
�����������ǶԻ�ѧ����ʽ�й�����Ŀ��飬����Ĺؼ�������Ӧ��Ҫ����й����ʵ��������ܽ��֪ʶ��Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
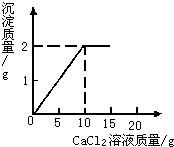 ��15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ�����������ϵ��ͼ��ʾ��
��15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ�����������ϵ��ͼ��ʾ��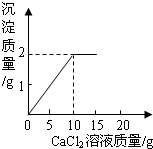 ��֪CaCl2+Na2CO3=CaCO3��+2NaCl����15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ��������ϵ��ͼ��ʾ��������Ӧ������ʹ��ˣ�������Һ��õ�����������Ƕ��٣�
��֪CaCl2+Na2CO3=CaCO3��+2NaCl����15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ��������ϵ��ͼ��ʾ��������Ӧ������ʹ��ˣ�������Һ��õ�����������Ƕ��٣� ��1��x��ֵΪ
��1��x��ֵΪ