��Ŀ����
ijУ��ʦ�û�ѧʵ��С���ͬѧ�����й�Na2CO3��NaCl��ϵ��ʵ�飮
ʵ��һ������ʧȥ��ǩ��Na2CO3��Һ��NaCl��Һ��
ͬѧ�Ƿֱ�ѡ�����������ʽ���ʵ�飮���к�������________��
��ϡ����ڳ���ʯ��ˮ��pH��ֽ
ʵ�������ȥijNaCl��Ʒ�е�����Na2CO3��
ͬѧ�����������ʵ������ͼ��
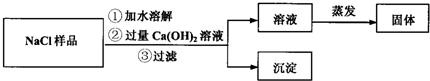
��1����������ͼ�У������Ļ�ѧʽΪ________��
��2��ʵ����Ϻ�ͬѧ�Ƿ�����Һ�е����ʳ�����NaCl�����________������ͬѧ�Ǹ������µķ���������Ʒ�еμ�ϡ���������ٲ�������Ϊֹ���йط�Ӧ�Ļ�ѧ����ʽ________��
ʵ���������������ʵ���ͬѧ���ֽ��������µ�̽�����
��ʵ��Ŀ�ġ��ⶨ��NaCl��Ʒ��Na2CO3������������
����Ʒ���������ʵ��Ŀ�ĺ�ԭ����ͬѧ����Ϊ��Ҫ��������ĸ����ݣ�
��NaCl��Ʒ������ag��
�ڳ�ַ�Ӧ�����ɳ���������Ϊbg��
�۷�Ӧǰװ�ú�ҩƷ������Ϊcg��
�ܳ�ַ�Ӧ��װ�ú�ҩƷ������Ϊdg��
��ʵ����Ʒ����ʦ�ṩ������ʵ����Ʒ��
ҩƷ��������ϡH2SO4��CaCl2��Һ����ʯ�ҡ�Ũ����
װ�ã�
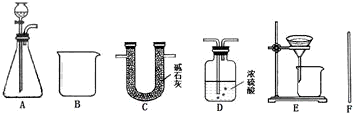
���������ϡ���CaCl2��Na2CO3������Һ�ܷ������ֽⷴӦ���ڼ�ʯ�����ռ�������ƵĹ��������Ҫ��������������
��ʵ�鱨�桿���������������±�����ʯ�Һ�Ũ�����ѱ�ѡ�ã��±��в�����д����
| ���� | ҩƷ | ʵװ�� | �������� ����ag��bg��cg��dg��ʾ�� | Na2CO3��������������ʽ |
| һ | ________ | D��E��F | ________ | ________ |
| �� | ________ | ________ | ag��cg��dg | ________ |
| �� | ________ | ________ | ________ | ________ |
���PH��7����NaCl��Һ�����ԣ�
ʵ�������1����������ͼ��֪��̼�����ܺ�����ʯ��ˮ��Ӧ���ɳ���������Ļ�ѧʽΪCaCO3��
��2��ʵ����Ϻ�ͬѧ�Ƿ�����Һ�е����ʳ�����NaCl�����Ca��OH��2��NaOH����Ϊ��Ʒ�е�Na2CO3��Ca��OH��2��Һ��Ӧʱ��ʯ��ˮ�ǹ����ģ�����������NaOH����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl�T2NaCl+H2O+CO2����
ʵ���������������ʵ��������µ�̽���������ʵ��̽����ȫ���̣�ʵ��Ŀ�ġ���Ʒ�����ʵ����Ʒ���������ϡ�ʵ�鱨����ʵ�鷴˼��
��ʵ�鱨������ɱ������£�
| ���� | ҩƷ | ʵװ�� | �������� ����ag��bg��cg��dg��ʾ�� | |
| һ | CaCl2��Һ | D��E��F | ag��bg |  % % |
| �� | ϡH2SO4 | A��D��C | ag��cg��dg | 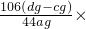 100% 100% |
| �� | �� ϡH2SO4 | ��A | ag��cg��dg | 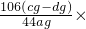 100% 100% |
ʵ��һ���٢ڢ�
ʵ�������1��CaCO3
��2��Ca��OH��2��NaOH
Na2CO3+2HCl�T2NaCl+H2O+CO2��
ʵ��������ע���±��з������ͷ��������Եߵ����ж��ַ�������
| ���� | ҩƷ | ʵװ�� | �������� ����ag��bg��cg��dg��ʾ�� | |
| һ | CaCl2��Һ | D��E��F | ag��bg |  % % |
| �� | ϡH2SO4 | A��D��C | ag��cg��dg | 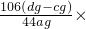 100% 100% |
| �� | �� ϡH2SO4 | ��A | ag��cg��dg | 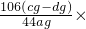 100% 100% |
������ʵ��һ���ɸ���̼���ƵĻ�ѧ���ʼ����ᷴӦ����������̼���塢�����ʯ��ˮ��Ӧ������������ˮ��Һ�Լ���������
ʵ�������1����������ͼ�У�������Ʒ�е�Na2CO3��Ca��OH��2��Һ��Ӧ�ȵý������2��������Ʒ�е�Na2CO3��Ca��OH��2��Һ��Ӧʱ��ʯ��ˮ�ǹ����ģ�����������NaOH����д��̼���������ᷴӦ�Ļ�ѧ����ʽ��
ʵ��������Ϥ����ʵ��̽����ȫ���̣�ʵ��Ŀ�ġ���Ʒ�����ʵ����Ʒ���������ϡ�ʵ�鱨����ʵ�鷴˼��������ʵ�鱨�����д���ǽ�������������̽���Ļ��������õ�ʵ��ɹ���
���������⿼�����ʧȥ��ǩ��Na2CO3��Һ��NaCl��Һ��̽���������漰֪ʶ��ḻ���ۺ���ǿ��ʵ��һ������ѧ�������ĺ��⣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| |||||||||||||||