��Ŀ����
 ʵ������-ƿ���ᣬ��ǩ��ͼ��ʾ��������ݱ�ǩ��ʾ�����ݻش��������⣺
ʵ������-ƿ���ᣬ��ǩ��ͼ��ʾ��������ݱ�ǩ��ʾ�����ݻش��������⣺
��1����300mL ���������������������Ϊ30%�����______g��
��2��������õ�����ⶨij���۽�����R����ͭ��ɵĺϽ���ͭ������������ȡ30g �úϽ𣬼�������ƺõ�ϡ����490g ��ϣ�ǡ����ȫ��Ӧ�����ˣ�����Һ���ɣ��õ�����171g����
�ٽ���R ͬ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ______
��������֪������ⷴӦ���ɵ�����������x���ı���ʽΪ______
�ۺϽ���ͭ����������Ϊ______
������Ӧ�����Һ�м���170g ˮ�����ʱ��Һ�����ʵ�����������______��
�⣺��1�������������������Ϊ30%�����������ΪX��
300ml��1.4g/cm3��50%=X��30%��
X=700g��
���700��
��2���ٽ���Rͬ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ��2R+3H2SO4�TR2��SO4��3+3H2����
���2R+3H2SO4�TR2��SO4��3+3H2����
��490gϡ�����к������������Ϊ��490g��30%=147g��
2R+3H2SO4�TR2��SO4��3+3H2����
294 6
147g x
 =
=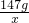 ��
��
x=3g��
��Ӧ���ɵ�����������x���ı���ʽΪ�� =
=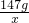 ��
��
��� =
=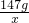 ��
��
�۽���Һ���ɣ��õ��Ĺ���171g��R2��SO4��3��������
�����R�����ԭ��������R��
2R+3H2SO4�TR2��SO4��3+3H2����
2R+288 6
171g 3g
 =
= ��
��
R=27��
171gR2��SO4��3��Ԫ��R������Ϊ��171g�� ��100%=27g��
��100%=27g��
R2��SO4��3��Ԫ��R�������ɽ���Rת�����������ԺϽ���ͭ������Ϊ��30g-27g=3g��
�Ͻ���ͭ����������Ϊ�� ��100%=10%��
��100%=10%��
���10%��
������Ӧ�����Һ�м���170g ˮ�����ʱ��Һ������Ϊ��27g+490g-3g+170g=684g��
���ʱ��Һ�����ʵ����������ǣ� ��100%=25%��
��100%=25%��
���25%��
��������1��ϡ��ǰ�������������䣻
��2��������Ŀ���ṩ�����ݿ��Խ�����ط���ļ��㣮
������������Ҫ�����ƶϺͼ��㷽�������������ʱҪ�������桢ȷ���ƶ�Ҫ�����۸��ݣ�
300ml��1.4g/cm3��50%=X��30%��
X=700g��
���700��
��2���ٽ���Rͬ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ��2R+3H2SO4�TR2��SO4��3+3H2����
���2R+3H2SO4�TR2��SO4��3+3H2����
��490gϡ�����к������������Ϊ��490g��30%=147g��
2R+3H2SO4�TR2��SO4��3+3H2����
294 6
147g x
 =
=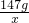 ��
��x=3g��
��Ӧ���ɵ�����������x���ı���ʽΪ��
 =
=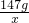 ��
�����
 =
=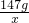 ��
���۽���Һ���ɣ��õ��Ĺ���171g��R2��SO4��3��������
�����R�����ԭ��������R��
2R+3H2SO4�TR2��SO4��3+3H2����
2R+288 6
171g 3g
 =
= ��
��R=27��
171gR2��SO4��3��Ԫ��R������Ϊ��171g��
 ��100%=27g��
��100%=27g��R2��SO4��3��Ԫ��R�������ɽ���Rת�����������ԺϽ���ͭ������Ϊ��30g-27g=3g��
�Ͻ���ͭ����������Ϊ��
 ��100%=10%��
��100%=10%�����10%��
������Ӧ�����Һ�м���170g ˮ�����ʱ��Һ������Ϊ��27g+490g-3g+170g=684g��
���ʱ��Һ�����ʵ����������ǣ�
 ��100%=25%��
��100%=25%�����25%��
��������1��ϡ��ǰ�������������䣻
��2��������Ŀ���ṩ�����ݿ��Խ�����ط���ļ��㣮
������������Ҫ�����ƶϺͼ��㷽�������������ʱҪ�������桢ȷ���ƶ�Ҫ�����۸��ݣ�

��ϰ��ϵ�д�
�����Ŀ
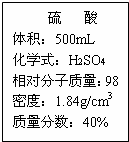 ��1����500mLŨ���������������������Ϊ20%������
��1����500mLŨ���������������������Ϊ20%������

 ��2013?�ɱ���һģ��ʵ��������ƿ��ͬ����ʧȥ��ǩ������Һ����Ϊ���г����Ļ�ѧ���ʣ��ֱ���̼�����Һ����������Һ��һ�ֺ��б����ӵ�δ֪��Һ��
��2013?�ɱ���һģ��ʵ��������ƿ��ͬ����ʧȥ��ǩ������Һ����Ϊ���г����Ļ�ѧ���ʣ��ֱ���̼�����Һ����������Һ��һ�ֺ��б����ӵ�δ֪��Һ��