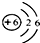��Ŀ����
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ���1������̼��������������������ģ���Ҫ��Ϊ�˼���
��2����ͼΪԪ�����ڱ��е�һ������˵������ȷ����
A��̼Ԫ�����ڷǽ���Ԫ��
B��̼ԭ�Ӻ���������Ϊ6
C��̼Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
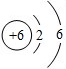
D��̼�����ԭ������Ϊ12.01
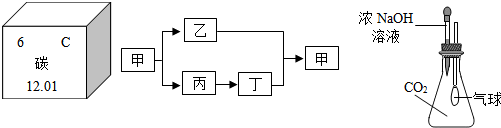
��3���ס��ҡ��������dz��л�ѧ�����Ļ���������к���̼Ԫ�أ������������������֮��������ͼ��ʾ��ת����ϵ���������ʺͷ�Ӧ��������ȥ������Ļ�ѧʽΪ
��4���ұ���ijȤζʵ��װ��ͼ����ѹ��ͷ�ιܺɹ۲쵽�����ʹ�������������ԭ��д����ѧ����ʽ
��5��������ͼװ�ÿ���CO��ԭFe2O3����ʵ�飬������÷�Ӧ���ɵ���������֪��Aװ����ȡ��CO�����л���������CO2��
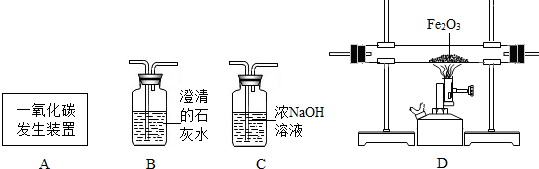
��CO��Fe2O3��Ӧ�Ļ�ѧ����ʽΪ
������ͨ��װ�õ�˳����A��
�۴ӻ����Ƕȿ��ǣ�������װ�õĸĽ���ʩ��
��6����ȡ12.5gʯ��ʯ����Ҫ�ɷ���CaCO3�����ʲ��μӷ�Ӧ�������ձ��У������м���50gϡ���ᣬ����ǡ����ȫ��Ӧ����Ӧ����������ձ���ʣ�����ʵ�������Ϊ58.1g���������ձ�����������������ܽ���Բ��ƣ����Լ���ʯ��ʯ�����ʵ�����������
��������̼������Ǽ��ٶ�����̼��̼�Ļ�������һ����̼��������̼��̼��Ƶȣ�����̼��̼�Ļ���������ʽ��н�𣮼��������̼�ó���ʯ��ˮ���������ǻ�ѧ�����г��õ�һ�ַ�����
����⣺��1������̼��������������������ģ���Ҫ��Ϊ�˼��� ������̼���ŷţ�
��2��̼ԭ�Ӻ��������ӦΪ4�����ӣ���C����
��3�������к���̼Ԫ�أ�������������������ĸ�����������ơ��������ơ�Ũ����ֽ�����Һͱ����ʱ��������ƣ�����̼��ƣ����Ƕ�����̼��������������ˮ�������ɵ��������ƣ�
��4��CO2��ŨNaOH��Һ���գ���ƿ�ڵ���ѹ��С��������ѹ������ƿ�ڵ���ѹ��ʹ�����ʹ� ��ѧ����ʽΪ 2NaOH+CO2=Na2CO3+H2O
��5��CO��Fe2O3��Ӧ�������Ͷ�����̼����ѧ����ʽΪ Fe2O3+3C0
2Fe+3CO2 ���������̼Ӧ�ó���ʯ��ˮ����˳��ΪC��D��B һ����̼�ж����ŷŵ������л���Ⱦ��������Ӧ��β����ȼ������ռ�������
��6����Ӧǰ���ձ��������ļ�������Ϊ���ɵĶ�����̼�����������ݻ�ѧ����ʽ�ж�����̼�����������̼��Ƶ�����������������ʵ�����������
�ʴ�Ϊ����1��CO2 ��2��C
��3��CaCO3 CaO+H2O=Ca��OH��2
��4��CO2��ŨNaOH��Һ���գ���ƿ�ڵ���ѹ��С��������ѹ������ƿ�ڵ���ѹ��ʹ�����ʹ� 2NaOH+CO2=Na2CO3+H2O
��5����Fe2O3+3C0
2Fe+3CO2 ��C��D��B
�۽�β����ȼ������ռ��ȣ�
��6���⣺����CO2������Ϊ��12.5 g+50 g-58.1g=4.4g
��ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
x 4.4g
=
��x=10g
ʯ��ʯ�����ʵ���������Ϊ��
��100%=20%
��ʯ��ʯ�����ʵ���������Ϊ20%��
��2��̼ԭ�Ӻ��������ӦΪ4�����ӣ���C����
��3�������к���̼Ԫ�أ�������������������ĸ�����������ơ��������ơ�Ũ����ֽ�����Һͱ����ʱ��������ƣ�����̼��ƣ����Ƕ�����̼��������������ˮ�������ɵ��������ƣ�
��4��CO2��ŨNaOH��Һ���գ���ƿ�ڵ���ѹ��С��������ѹ������ƿ�ڵ���ѹ��ʹ�����ʹ� ��ѧ����ʽΪ 2NaOH+CO2=Na2CO3+H2O
��5��CO��Fe2O3��Ӧ�������Ͷ�����̼����ѧ����ʽΪ Fe2O3+3C0
| ||
��6����Ӧǰ���ձ��������ļ�������Ϊ���ɵĶ�����̼�����������ݻ�ѧ����ʽ�ж�����̼�����������̼��Ƶ�����������������ʵ�����������
�ʴ�Ϊ����1��CO2 ��2��C
��3��CaCO3 CaO+H2O=Ca��OH��2
��4��CO2��ŨNaOH��Һ���գ���ƿ�ڵ���ѹ��С��������ѹ������ƿ�ڵ���ѹ��ʹ�����ʹ� 2NaOH+CO2=Na2CO3+H2O
��5����Fe2O3+3C0
| ||
�۽�β����ȼ������ռ��ȣ�
��6���⣺����CO2������Ϊ��12.5 g+50 g-58.1g=4.4g
��ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
x 4.4g
| 100 |
| 44 |
| x |
| 4.4g |
ʯ��ʯ�����ʵ���������Ϊ��
| 12.5g-10g |
| 12.5g |
��ʯ��ʯ�����ʵ���������Ϊ20%��
�����������ۺϿ�����̼��̼�Ļ���������ʼ����㣬�ۺ���ǿ��Ҫ��ѧ���������ջ���֪ʶ���ܺܺõĽ���⣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ� ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ� D��̼�����ԭ������Ϊ12.01
D��̼�����ԭ������Ϊ12.01
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�