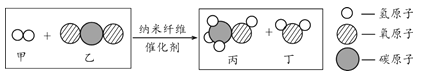
��Ŀ����
����Ŀ��ijУѧ��ѧϰ������������̽����
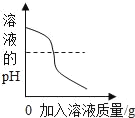
����һ����AgNO3��Һ��������ͼʵ�顣
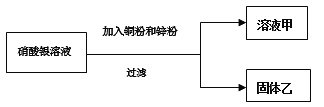
��.��������ϵμ�����û�����ݲ�����������ҵ������___________��
��.��Һ�׳���ɫ����Һ����һ������������_________________��ʹ��Һ����ɫ�Ļ�ѧ��Ӧ��____________________��д��ѧ����ʽ�����÷�Ӧ�Ļ��������� _______________________��
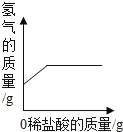
�ڳ�ȡͭ�ۺ�п�ۻ����10.0�ˣ���ε���100��ϡ������Һ��ʵ�������ͼ��ʾ��
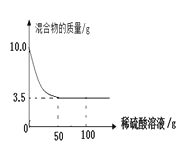
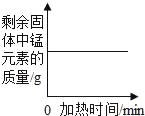
��.�������Zn�����ʵ�����________mol��
��.ϡ������Һ�����ʵ���������_________��
���𰸡� Ag��Ag��Cu Cu(NO3)2��Zn(NO3)2 Cu + 2AgNO3 ��Cu(NO3)2 + 2Ag �û���Ӧ 0.1 19.6%
����������ʵ�����Һ�׳���ɫ��˵������ͭ�Σ���п�۲��㣬�����������ϵμ�����ʱû�����ݲ������ɽ������˳�������ÿ�֪���������Al��Zn��Cu��Ag������п��������AgNO3������Ӧ��ͭ�ۺ���AgNO3������Ӧ������п�����IJ��㣬û�а�����ͭ��Һ�е�ͭ�û������������Һ��һ�����з�Ӧ���ɵ�Zn ��NO3��2��ʣ���Cu��NO3��2�����ܺ���AgNO3��������һ�����б��û������������ܺ���ͭ�������Ϸ�����֪��
������п���㣬�����������ϵμ�����ʱû�����ݲ������������н����ijɷ�����������ͭ��
����Һ����һ�����е�������Cu��NO3��2��Zn��NO3��2��ʹ��Һ����ɫ�Ļ�ѧ��Ӧ��Cu+2AgNO3=Cu��NO3��2+2Ag��д��ѧ����ʽ�����÷�Ӧ�Ļ����������û���Ӧ��
�ڢ��ɷ�Ӧ��ʵ�����ͼ����ȫ��Ӧ��ʣ����������Ϊ3.5g����ͭп�Ͻ���Ʒ�к�ͭ������Ϊ3.5g����п������Ϊ10g-3.5g=6.5g���ʻ������Zn�����ʵ�����![]() =0.1mol��
=0.1mol��
���跴Ӧ������ϡ������Һ�����ʵ�����Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 98
6.5gx
![]()
���x=9.8g
����ϡ������Һ�����ʵ���������Ϊ�� ![]() ��100%=19.6%��
��100%=19.6%��
������ϡ������Һ�����ʵ�����������19.6%��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�����Ŀ���±�������ʵ�������ijЩ��֮��ı仯��ϵ��������ȷ���ǣ�������
�� | �� | �� | �� |
��һ���������еμ�ϡ���� | ����һ����������ع��� | ��ϡ�����еμӹ���������������Һ | ������ز�������Һ��������ˮ�֣�ֱ���о������� |
|
|
|
|
A. ֻ�Т�B. ֻ�Т�C. �١��ڡ���D. �ڡ��ۡ���
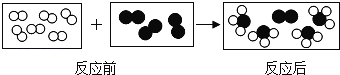
����Ŀ��ijѧϰС����̽����Ļ�ѧ����ʱ��������ͼ��ʾ������ʵ�飺
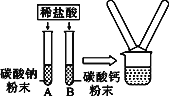
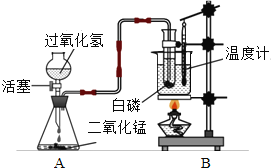
��1���ֱ�����֧װ��̼���ƺ�̼��Ʒ�ĩ���Թ���ע��һ������ϡ���ᣬ��֧�Թ��ھ��۲쵽��������___________�������ĩȫ����ʧ�������γ���ɫ��Һ��
��2��ʵ�������ͬѧ�ǽ�A��B��֧�Թ��еķ�Һ����ͬһֻ�ྻ���ձ��У������ձ��еķ�Һ����ǡ����ˣ�����Һ�е����ʳɷֽ���̽��������һ����롣
��������룩
����1����Һ�е��������Ȼ��ƣ�
����2����Һ�е��������Ȼ��ƺ�̼���ƣ�
����3����Һ�е�������___________��
ʵ�鲽�� | Ԥ��ʵ������ | Ԥ�ƽ��� |
ȡ��������Һ���Թ��У�______ | ______ | ����______��ȷ |
�����ʵ�飩����ѡ��һ�ֲ��������֤��