��Ŀ����
 �������Ƽ���ƵõĴ�����ͨ�������������Ȼ��ƣ�ijͬѧ��ⶨ���������Ȼ������ʵĴ�����Ʒ��̼���ƣ�Na2CO3����������������ͬѧ�������£��ֳ�ȡ6g���������ձ��в�����ϡ���ᣬ��ϡ����μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ�����ʴ����⣺
�������Ƽ���ƵõĴ�����ͨ�������������Ȼ��ƣ�ijͬѧ��ⶨ���������Ȼ������ʵĴ�����Ʒ��̼���ƣ�Na2CO3����������������ͬѧ�������£��ֳ�ȡ6g���������ձ��в�����ϡ���ᣬ��ϡ����μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ�����ʴ����⣺��1�����������غ㶨�ɣ����A��������������Ϊ
��2��������̼���Ƶ���������
��3��B��ʱ���ձ�����Һ�����ʵĻ�ѧʽΪ
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1����ͼ��֪��A��ʱ�����̼����ǡ����ȫ��Ӧ����������������������ձ��е�����ǰ����ٵ�������
��2������̼���������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼���������̼���Ƶ��������ٸ������������ļ��㹫ʽ���������̼���Ƶ�����������
��3����B��ʱ��̼�����ѷ�Ӧ�꣬����ʣ������ᣬ�����ʱ��Һ�е����ʳ��˷�Ӧ���ɵ��������ʣ��������е����ʣ�
��2������̼���������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼���������̼���Ƶ��������ٸ������������ļ��㹫ʽ���������̼���Ƶ�����������
��3����B��ʱ��̼�����ѷ�Ӧ�꣬����ʣ������ᣬ�����ʱ��Һ�е����ʳ��˷�Ӧ���ɵ��������ʣ��������е����ʣ�
����⣺��1��A��������������Ϊ��6g+36.5g-40.3g=2.2g��
��2����6g������Na2CO3����Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 2.2g
=
������Na2CO3����������=
��100%��88.3%
��3����̼���ƺ�����ǡ����ȫ��Ӧʱ����Һ�е��������Ȼ��ƣ�����B��ʱ�����ѹ�������Һ�е����ʳ������Ȼ�����������е������Ȼ��⣻�ձ�����Һ��������Ϊ��40.3g+��73g-36.5g��=76.8g��
�ʴ�Ϊ����1��2.2��
��2��88.3%��
��3��NaCl��HCl��76.8��
��2����6g������Na2CO3����Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 2.2g
| 106 |
| x |
| 44 |
| 2.2g |
������Na2CO3����������=
| 5.3g |
| 6g |
��3����̼���ƺ�����ǡ����ȫ��Ӧʱ����Һ�е��������Ȼ��ƣ�����B��ʱ�����ѹ�������Һ�е����ʳ������Ȼ�����������е������Ȼ��⣻�ձ�����Һ��������Ϊ��40.3g+��73g-36.5g��=76.8g��
�ʴ�Ϊ����1��2.2��
��2��88.3%��
��3��NaCl��HCl��76.8��
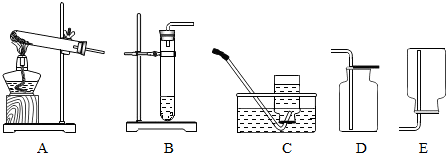
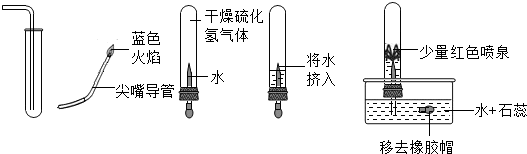
������������Ҫ�����˸���ͼ�������֪�������ݻ�ѧ����ʽ������ط�����㣮

��ϰ��ϵ�д�
�����Ŀ
 ����
���� ���ֱ��ʾ��ͬ�����ԭ�ӣ�������ʾ��ͼ�п��Ա�ʾ��������ǣ�������
���ֱ��ʾ��ͬ�����ԭ�ӣ�������ʾ��ͼ�п��Ա�ʾ��������ǣ�������