��Ŀ����
���ᾧ�壨H2C2O4?2H2O���۵�ϵͣ�����ʱ��Ѹ���ۻ��������ֽ⣬ijʵ��С��Ϊ�������ֽ�������Ƿ���CO���������ͼ��ʾ��ʵ��װ�ã��ش������й����⣺
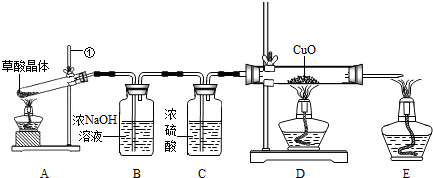
��1��д��Aװ���������ٵ����� ��
��2��֤������CO������ ��D�з�Ӧ�Ļ�ѧ����ʽ�� ��
��3��Bװ�õ������� ��
��4��E���ƾ��Ƶ������� ��ʵ�����ʱ��Ӧ��Ϩ�� �����A������D����E�����ľƾ��ƣ�
��5��Ϊ�ⶨij���������ʵ�����������С��ͬѧȡ��������Ʒ50g�������м������п������ɷ�Ӧ���ռ���0.3g��������������м������������������������ʵ�������������С�ٽ��ⶨ����������Լ�ƿ��ǩ������������Ϣ��Ũ���ᡢ���ʵ���������Ϊ35%�����жԱȣ����ֱ�ǩ�����ʵ�����������ʵ�ʲ�õ�ֵ������ʵ�������������Ϊԭ������� ��

��1��д��Aװ���������ٵ�����
��2��֤������CO������
��3��Bװ�õ�������
��4��E���ƾ��Ƶ�������
��5��Ϊ�ⶨij���������ʵ�����������С��ͬѧȡ��������Ʒ50g�������м������п������ɷ�Ӧ���ռ���0.3g��������������м������������������������ʵ�������������С�ٽ��ⶨ����������Լ�ƿ��ǩ������������Ϣ��Ũ���ᡢ���ʵ���������Ϊ35%�����жԱȣ����ֱ�ǩ�����ʵ�����������ʵ�ʲ�õ�ֵ������ʵ�������������Ϊԭ�������
���㣺ʵ��̽�����ʵ���ɳɷ��Լ�����,��������ļ�������ӷ���,һ����̼�Ļ�ѧ����,�й��������������ļ���,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
��������1������ʵ���ҳ���������������գ�
��2������һ����̼���л�ԭ�ԣ���ʹ�������������ɽ������ʽ��
��3����������������Һ�ܺͶ�����̼��Ӧ����̼���ƺ�ˮ���н��
��4������һ����̼�ж��Լ�һ����̼��ԭ����ͭע��������н��
��5��������������������������������ʵ����������Լ�Ũ������лӷ��Խ��н��
��2������һ����̼���л�ԭ�ԣ���ʹ�������������ɽ������ʽ��
��3����������������Һ�ܺͶ�����̼��Ӧ����̼���ƺ�ˮ���н��
��4������һ����̼�ж��Լ�һ����̼��ԭ����ͭע��������н��
��5��������������������������������ʵ����������Լ�Ũ������лӷ��Խ��н��
����⣺��1��ͼ��Aװ���������ٵ�����������̨��������е�����̨����
�ʴ��ǣ�����̨��������е�����̨����
��2��һ����̼�Ļ�ԭ���ܺͽ��������ﷴӦ�����ɽ����Ͷ�����̼��������һ����̼��ԭ����ͭ��ʵ���У�������ͭΪ��ɫ��ͭΪ��ɫ������ʵ���пɹ۲쵽D����ɫ��ĩ��죬D�з�Ӧ�Ļ�ѧ����ʽ��CuO+CO
Cu+CO2��
�ʴ�Ϊ����ɫ������ɺ�ɫ��CuO+CO
Cu+CO2��
��3������������Һ�ܺͶ�����̼��Ӧ����̼���ƺ�ˮ������Bװ�õ������dz�ȥһ����̼�����еĶ�����̼��
�ʴ��ǣ���ȥһ����̼�����еĶ�����̼��
��4��������ͭ��ȫ��Ӧʱ����Ҫ����ͨһ����̼��ֱ���Թ���ȴΪֹ������Ϩ��ƾ���ʱҪ��Ϩ��D���ƾ������Ϩ��E���ƾ��ƣ�E���ƾ��Ƶ����þ��ǵ�ȼδ��Ӧ��һ����̼ʹ��ת��Ϊ������̼����ֹ��Ⱦ������
�ʴ��ǣ���ȼδ��Ӧ��һ����̼ʹ��ת��Ϊ������̼����ֹ��Ⱦ������D��
��5������50g�������Ȼ��������Ϊx��
Zn+2HCl�TZnCl2+H2��
73 2
x 0.3g
=
x=10.95g
�����������ʵ���������Ϊ��
��100%=21.9%
�𣺸����������ʵ���������Ϊ21.9%��
����ΪŨ������лӷ��ԣ������Ȼ���ӷ���ᵼ����������������С��
���Ũ������лӷ��ԣ����ʻӷ�������������������С��
�ʴ��ǣ�����̨��������е�����̨����
��2��һ����̼�Ļ�ԭ���ܺͽ��������ﷴӦ�����ɽ����Ͷ�����̼��������һ����̼��ԭ����ͭ��ʵ���У�������ͭΪ��ɫ��ͭΪ��ɫ������ʵ���пɹ۲쵽D����ɫ��ĩ��죬D�з�Ӧ�Ļ�ѧ����ʽ��CuO+CO
| ||
�ʴ�Ϊ����ɫ������ɺ�ɫ��CuO+CO
| ||
��3������������Һ�ܺͶ�����̼��Ӧ����̼���ƺ�ˮ������Bװ�õ������dz�ȥһ����̼�����еĶ�����̼��
�ʴ��ǣ���ȥһ����̼�����еĶ�����̼��
��4��������ͭ��ȫ��Ӧʱ����Ҫ����ͨһ����̼��ֱ���Թ���ȴΪֹ������Ϩ��ƾ���ʱҪ��Ϩ��D���ƾ������Ϩ��E���ƾ��ƣ�E���ƾ��Ƶ����þ��ǵ�ȼδ��Ӧ��һ����̼ʹ��ת��Ϊ������̼����ֹ��Ⱦ������
�ʴ��ǣ���ȼδ��Ӧ��һ����̼ʹ��ת��Ϊ������̼����ֹ��Ⱦ������D��
��5������50g�������Ȼ��������Ϊx��
Zn+2HCl�TZnCl2+H2��
73 2
x 0.3g
| 73 |
| x |
| 2 |
| 0.3g |
x=10.95g
�����������ʵ���������Ϊ��
| 10.95g |
| 50g |
�𣺸����������ʵ���������Ϊ21.9%��
����ΪŨ������лӷ��ԣ������Ȼ���ӷ���ᵼ����������������С��
���Ũ������лӷ��ԣ����ʻӷ�������������������С��
������������Ҫ�������ʵĻ�ѧ���ʼ���ѧ����ʽ����д��Ҫ��ѧ���������õ�ʵ����������ͽ���������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
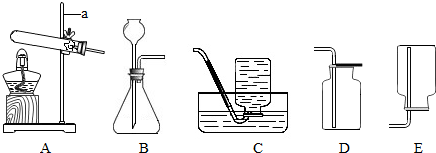
��ʢŨ�����ƿ�������Կ���ƿ���У�������
| A���Ȼ������� | B������ |
| C������ | D����ɫ������ |
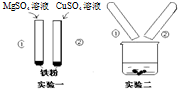 ij��ѧ��ȤС���ͬѧ��̽��Mg��Cu��Fe���ֽ������й�����ʱ����������ʵ�飺
ij��ѧ��ȤС���ͬѧ��̽��Mg��Cu��Fe���ֽ������й�����ʱ����������ʵ�飺 ��
�� �ֱ��ʾ��ԭ�Ӻ���ԭ�ӣ���ش��������⣺
�ֱ��ʾ��ԭ�Ӻ���ԭ�ӣ���ش��������⣺
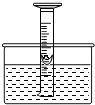 ��ͼ��Ϊ���о������������������������һ��50mL��Ͳ������ˮ�У���Ͳ����Һ���λ��40mL������Ͳ�ڸ���һ��ͭ�Ƶ�С�������з����������ף��ְ��������ƻ���Ͷ��ˮ�в����裬��������ԭ����
��ͼ��Ϊ���о������������������������һ��50mL��Ͳ������ˮ�У���Ͳ����Һ���λ��40mL������Ͳ�ڸ���һ��ͭ�Ƶ�С�������з����������ף��ְ��������ƻ���Ͷ��ˮ�в����裬��������ԭ���� ���͡�
���͡� ����ʾ��ͬԪ�ص�ԭ�ӣ�������ʾ��ͼ�ܱ�ʾ���������
����ʾ��ͬԪ�ص�ԭ�ӣ�������ʾ��ͼ�ܱ�ʾ���������