��Ŀ����
����Ŀ����У��ȤС�鼸λͬѧ����ѧϰ�˾��꼶��ѧ������Ԫ���ܲ���������̼��һЩ���ʲ�������Ȥ��
���м�λͬѧ��ʵ���ҵIJ��ᣨC2H2O4����������Ȥ�����������ʦ��֪������Ļ�ѧ������̼�����ơ�̼���ֽ⣬����������������Ҳ�ֽ⣬������������������ǶԴ�չ����̽����
��1��Сٻ���ȸ��ݲ����к���________Ԫ�أ����Ʋ��������к���ˮ��
��2��Сٻ�Էֽ����ɵ�����������в²⡢��֤��
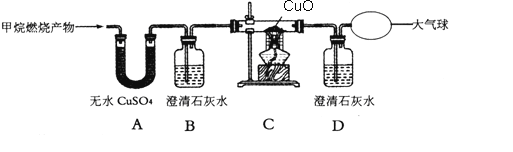
������һ�����������ж�����̼
ʵ��һ�������ɵ�����ͨ������ʯ��ˮ��ʵ��������______����Ӧ����ʽΪ__________С���ݴ��ж��������к��ж�����̼��
�������������������һ����̼
ʵ�����Сܿ����ͼװ�ý���ʵ�飬�Ӷ�֤���������к���һ����̼��
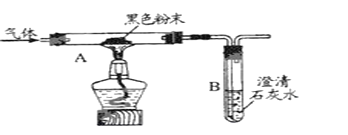
��ش�Aװ���к�ɫ�����ĩ��______ (�ѧʽ)��
��3�����۽�����С����Ϊ��Сܿ�ġ�ʵ���������ҪBװ��Ҳ�ܹ��ƶϳ��������к���һ����̼�����Ƿ�֧��С���Ĺ۵㣺________(�֧�֡���֧�֡�)����˵��������ɣ�______��С����Ϊ���ӻ����ĽǶȿ�����ʵ�����װ����һ��ȱ�ݣ�����ָ����ȱ�ݣ�____________��
��4����ȤС�����λ��ͬѧ��ѧУ�����һ��ʯ��ʯ��������Ȥ��������֪�����к�̼��Ƶ�������������������ȡ����һЩʯ��ʯ����ȡϡ����200g��ƽ���ֳ�4�ݣ�ÿ�˽�����һ��ʵ�飬������£�
ʵ�� | 1 | 2 | 3 | 4 |
������Ʒ������/g | 5 | 10 | 15 | 20 |
����CO2������/g | 1.54 | 3.08 | 4.4 | m |
��m����ֵ��__________��
������Э�����Ǽ�������ʯ��ʯ��̼��Ƶ���������________����д�������ļ�����̣�
���𰸡������ ����ʯ��ˮ����� CO2+Ca(OH)2==CaCO3��+H2O CuO ֧�� ֻҪ��������ͭ�ɺ�ɫ��Ϊ��ɫ���Ϳ��жϲ����к���һ����̼ û��β������װ�ã���װ�õ�������Ӿƾ��� 4.4 70%
��������
��1����ѧ��Ӧǰ��Ԫ�ص�����䣬���ݲ����к�����Ԫ�ء���Ԫ�أ����Ʋ��������к���ˮ��
��2������һ��������̼�������ʯ��ˮ��Ӧ����̼��Ƴ�����ˮ�����Խ����ɵ�����ͨ�����ʯ��ˮ��ʵ�������dz���ʯ��ˮ����ǡ��������һ����̼���л�ԭ�ԣ��ܽ���ɫ������ͭ��ԭΪ��ɫ��ͭ��
��3��С���Ĺ۵�����ȷ�ģ�������̼�����л�ԭ�ԣ�ֻҪ�Ǻ�ɫ��ĩ��ɺ�ɫ����˵�����ڻ�ԭ�����ʣ���˵������һ����̼��β���к���һ����̼��һ����̼����Ⱦ������Ӧ����װ�ú�����һ���ƾ���ȼ�յ�β���е�һ����̼��
��4���⣺����ʵ�����ݷ�����֪��һ��ʵ����Ʒ�е�̼�����ȫ�μ��˷�Ӧ����һ��ʵ����Ʒ��̼��Ƶ�����Ϊx��
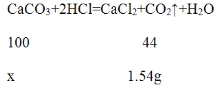
![]() =
=![]() �����x=3.5g
�����x=3.5g
ʯ��ʯ��̼��Ƶ���������Ϊ��![]() ��100%=70%
��100%=70%

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�����Ŀ����ʡ��������ú̿��Դ�ḻ����˹������ú�㼰��Χ�Ҳ��У��Ǿ����к�������ܳƣ���Ҫ�ɷ��Ǽ��飮
(1)����д��������ȫȼ�յĻ�ѧ����ʽ��_____
(2)ú����˹��ը��������Ҫ����������˹�����ڱ�ը���ķ�Χ�ڣ���____��
(3)�±��dz�����������ı�ը���ޣ�����ݴ��жϣ�
��ȼ���� | ��ը����(�������) |
H2 | 4.0%��74.2% |
CH4 | 5%��15% |
CO | 12.5%��74.2% |
����������ը��������_____��
(4)ij�ִ����Ŀ���������Ũ����˹���缼��������Ч����˿������ܱߵ����������õ磮���ּȲ�ú�ַ��������������ŵ���____��