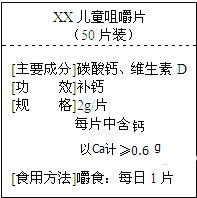
��Ŀ����
����Ŀ����һ����ɫ��ĩ��������Ca��OH��2��Na2CO3��BaCl2��Na2SO4��NaOH��CuSO4�е�һ�ֺͼ�����ɣ�Ϊ��̽������ijɷ֣���������ʵ�飺
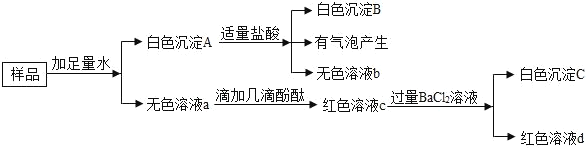
��1��ԭ������һ��û��_____��
��2��ԭ������һ����_____��
��3����������Ļ�ѧ����ʽ_____��
��4����ɫ��Һa��һ�����е�����_____��
��5�����й�����Һ�Ͱ�ɫ���������룬��������_____��
A����ȥ���ᱵ�е�����ð�ɫ����A
B����ɫ��Һa�ܳ�ȥ�Ȼ���������������
C�����������̼���ƣ�����Һb��c��d��
���𰸡�����ͭ Na2CO3��BaCl2��Na2SO4 BaCO3+2HCl=BaCl2+H2O+CO2�� NaCl��NaOH C
��������
����ͭ��Һ����ɫ����Ʒ��ˮ����ɫ��Һ����һ������������ͭ��̼�ᱵ�����������ᷴӦ����Һ�����ᱵ�������ܡ�ʵ���а�ɫ�����������ܲ������ݣ���һ����̼�ᱵ�������ʰ�ɫ������һ������̼���ƺ��Ȼ�����������������Ȼ���а�ɫ�������ʳ���һ�������ᱵ���ʹ�����һ�����������ƣ���ɫ��Һ�ӷ�̪��죬˵�����м������ʣ����Ȼ����ܲ�����ɫ����������Һ��Ϊ��ɫ��˵�������������ơ�
��1�������ƶϣ�ԭ������һ������������ͭ��
��2�������ƶϣ�ԭ������һ���� Na2CO3��BaCl2��Na2SO4��
��3����������Ļ�ѧ����ʽΪBaCO3+2HCl=BaCl2+H2O+CO2����
��4����ΪNa2CO3��BaCl2��Ӧ�����Ȼ��ƺ�̼�ᱵ��������ɫ��Һa��һ�����е����� NaCl�����������Ȼ�������Һ��ȻΪ��ɫ��˵����Һ�л���NaOH��
��5��A����ɫ����A���ܺ���̼��ƣ����������и����ӣ�ѡ�����
B����ɫ��Һa�п��ܴ��ڸ����ӣ����������и����ӣ������µ����ʣ�ѡ�����
C������ϡ�����̼������Һ������Һb��c��d��b�к����Ȼ���������̼���Ʒ�Ӧ������ɫ���������������Ȼ�������Ӧ��c��d��Һ���м�����Һ�����ᷴӦ������Һ���ɺ�ɫ�����ɫ��̼������Һ���ܸı���Һ������ԣ���Һ��Ϊ��ɫ�����Լ���
��ѡC��