��Ŀ����
����Ŀ����11����ij��ѧ��ȤС������ʦָ������ȡ������ռ���������̽��������һЩ���ʡ�
���Ͽ�Ƭ������ͨ����������д̼�����ζ����ɫ���壬�ܶȱȿ���С����������ˮ����Һ����Һ�������������
������ʯ�Һ��Ȼ�粒�������ķ����������ɰ�������Ҫ���ٲ������������Խ�Ũ��ˮ�μӵ���ʯ���еõ���
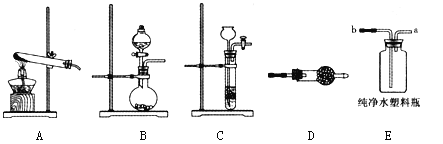
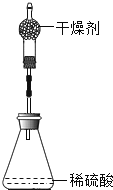
��1������Aװ����ȡ�����Ļ�ѧ����ʽΪ____����Ũ��ˮ�μӵ���ʯ�ҿ��ٲ�������������װ�����ѡ��____����װ�ñ��������д����ʯ���ڴ˷�Ӧ�е��������ã�____��____��
��2�����ﰱ�����Խ�����ͨ��װ��____���ѧʽ����װ��D��
��3����ȤС��ƻ���Eװ���հ����������жϰ����Ѿ������ķ�����_________��
��4������ͼ���������ƻ��ڴ���ˮ����ƿ��������Ȫ������ȡ�����IJ���?__________��
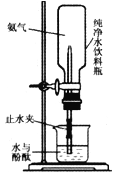
��5��ʵ���Ϊ��������ˮ����ƿ�ڴ��ڵİ�ˮ��NH3��H2O�����Լ�������ϡ���ᣬ������Ӧ�Ļ�ѧ����ʽΪ________________��
���𰸡���1��Ca��OH��2+��NH4��2SO4![]() CaSO4+2H2O+2NH3�� ��2���� B��1����
CaSO4+2H2O+2NH3�� ��2���� B��1����
CaO��ˮ��Ӧ���������ܼ���1����
CaO��ˮ��Ӧ�����з��ȣ�������NH3���ܽ�� ��1�֣��𰸺���������
��2��CaO����NaOH�� ��1����
��3����ʪ��ĺ�ɫʯ����ֽ����b�ˣ�����ֽ����ɫ��˵�������Ѿ�������2������ע�⣺��ԡ�b���˹۲�����l�֣���Ժ��ʵ��Լ��硰ʪ���pH��ֽ�����з�̪����������l����
��4����ѹ����ƿ��ʹ��������ˮ����1�֣��𰸺���������
��5��2NH3��H2O+H2SO4===��NH4��2SO4+2H2O��2����
��������
�����������1��������ʯ�Һ��Ȼ�粒�������ķ����������ɰ�������ѧ����ʽΪ��Ca��OH��2+��NH4��2SO4��CaSO4+2H2O+2NH3��������װ�õ�ѡ�������ǣ���Ӧ���״̬�ͷ�Ӧ��������Ũ��ˮ�μӵ���ʯ�ҿ��ٲ����������ǹ����Һ���ڳ����·�Ӧ������ѡB����ʯ���ڴ˷�Ӧ�е��������ã�1��CaO��ˮ��Ӧ���������ܼ���2��CaO��ˮ���ȣ�������NH3���ܽ��
��2����Ϊ��������ˮ�ʼ��ԣ����Բ�����Ũ���������ֻ���ü��Ը��������װ��DӦװCaO��NaOH
��3����Eװ���հ��������ڰ������ܶȱȿ���С��������Ӧ��a�˽���b�˳������жϰ����Ѿ������ķ�������ʪ��ĺ�ɫʯ����ֽ����b�ˣ�����ֽ����ɫ��˵�������Ѿ�����
��4�����ڼ�������ˮ�����Լ�ѹ����ƿ��ʹ��������ˮ�У�����ƿ�ڵ�ѹǿѸ�ټ��٣�������γ�ѹǿ�������Ȫ
��5��Ϊ��������ˮ����ƿ�ڴ��ڵİ�ˮ��NH3H2O�����Լ�������ϡ���ᣬ������Ӧ�Ļ�ѧ����ʽΪ��2NH3H2O+H2SO4 =��NH4��2SO4+2H2O

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�����Ŀ��С�����ֶ������̴���������ֽ��ʵ���У���Ӧ�ٶ��������������ϵ�֪����ԭ���ǹ����������������������С�Լ��������̵Ĵ��������͡�Ϊ��̽���������̵Ĵ��������͵�Ӱ�����أ���������ʵ�顣
����������ȡ������Ϊ2�˵Ķ������̷ֱ��40����������������Ϊ10����20����30���Ĺ���������Һ�ֽ⣬ʵ�����ն������̣����ֱ��ΪA��B��C;��ȡ��δ��ֽ�10������������Һʵ�����յĶ������̣���ΪD��
��ʵ�鲽�衿
������װ�ò���������ԡ�
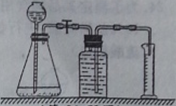
��ȡδʹ�ù��Ķ�������0��5�ˣ�������ƿ�У��ټ���10����������������Ϊ5���Ĺ���������Һ����¼�ռ�120�������������ʱ�䡣
���ڷֱ�ȡA��B��C��D���������0��5�ˣ��ظ�����ʵ�顣
�ܶ��ʵ��ȡƽ��ֵ����¼�������£�
��� | δʹ�ù��ö������� | A | B | C | D |
�ռ�����ʱ�䣯�� | 17.9 | 23.2 | 27.7 | 32.3 | 37.8 |
��1��ʵ��ʱ������©���¶˹ܿ�Ӧ��Һ�����£���Ŀ���� ��
��2��ʵ����ͨ���Ƚ��ռ���ͬ������������ʱ���⣬������ͨ���Ƚ� ����ӳ�������̵Ĵ�������
��3�����ϱ����ݿ�֪��������յĶ������̵Ĵ������½��������� ��