��Ŀ����
��2009?��ƽ��һģ������ͭ�����þ������п�е�һ����ɵĻ��������ⶨ����ɣ����������ϣ�þ������п������ϡ���ᷢ���û���Ӧ��������+2�۵Ŀ����Խ����������������
��ʵ�鲽�輰���ݣ�ȡ�û�����ĩ8.0g�����ձ��У���140.0g ���ʵ���������Ϊ14.0%��ϡ����ƽ�����Ĵμ�����ձ��У���ַ�Ӧ���ʣ������������ݼ�¼��ͼ��
ͨ�����㣨д��������̣�����
��1���û�����ĩ��ͭ������������
��2�������μ��������ַ�Ӧ��������Һ�����ʵ����������Ƕ��٣�

���𰸡���������ǰ��������ϡ��������ʣ����������ɵó���ÿ�μ���ϡ�����ַ�Ӧ�������������1.2g������4�μ������ϡ�����ַ�Ӧ���������ֻ����4.4g-4.2g=0.2gС��1.2g��˵������������ϡ���ᷴӦ�Ľ�������ȫ��Ӧ��ʣ���ȫ��Ϊ���������ᷴӦ�Ľ���ͭ��
���ݽ������ᷴӦ�Ļ�ѧ����ʽ�������Ľ������������������������������ԭ���������жϽ���Ԫ�أ�����������������������ɵ������ε���������Ӧ�ų��������������������������Һ�����ʵ���������= ×100%���������Һ���������������з�Ӧ����Һ���������������غ㶨�������
×100%���������Һ���������������з�Ӧ����Һ���������������غ㶨�������
����⣺��1���������Cu����������=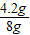 ×100%=52.5%
×100%=52.5%
��2����M�������ʵ����ԭ������ΪX��������Һ��MSO4������Ϊy������H2������Ϊz
��3�μ���ϡ�����ַ�Ӧ��ǰ���ι����Ľ���������=8.0g-4.4g=3.6g��
ǰ���ι��������������=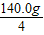 ×3×14%=14.7g
×3×14%=14.7g
M+H2SO4�TMSO4+H2����1�֣�
X 98 X+96 2
3.6g 14.7g y z
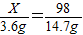 X=24�����жϸý�����þ��
X=24�����жϸý�����þ��
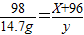 y=18g
y=18g
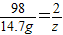 z=0.3g
z=0.3g
MSO4��Һ����������Ϊ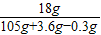 ×100%��16.6%
×100%��16.6%
�𣺸û�����ĩ��ͭ������������52.5%�������μ��������ַ�Ӧ��������Һ�����ʵ�����������16.6%��
��������Ӧ��������Һ�������ɸ��������غ㶨�ɽ��м��㣬��Ӧ����Һ������=ǰ��������ϡ���������+ǰ���η�Ӧ���Ľ���������-��Ӧ�ų�������������
���ݽ������ᷴӦ�Ļ�ѧ����ʽ�������Ľ������������������������������ԭ���������жϽ���Ԫ�أ�����������������������ɵ������ε���������Ӧ�ų��������������������������Һ�����ʵ���������=
 ×100%���������Һ���������������з�Ӧ����Һ���������������غ㶨�������
×100%���������Һ���������������з�Ӧ����Һ���������������غ㶨�����������⣺��1���������Cu����������=
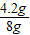 ×100%=52.5%
×100%=52.5%��2����M�������ʵ����ԭ������ΪX��������Һ��MSO4������Ϊy������H2������Ϊz
��3�μ���ϡ�����ַ�Ӧ��ǰ���ι����Ľ���������=8.0g-4.4g=3.6g��
ǰ���ι��������������=
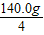 ×3×14%=14.7g
×3×14%=14.7gM+H2SO4�TMSO4+H2����1�֣�
X 98 X+96 2
3.6g 14.7g y z
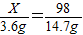 X=24�����жϸý�����þ��
X=24�����жϸý�����þ��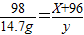 y=18g
y=18g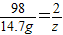 z=0.3g
z=0.3gMSO4��Һ����������Ϊ
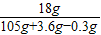 ×100%��16.6%
×100%��16.6%�𣺸û�����ĩ��ͭ������������52.5%�������μ��������ַ�Ӧ��������Һ�����ʵ�����������16.6%��
��������Ӧ��������Һ�������ɸ��������غ㶨�ɽ��м��㣬��Ӧ����Һ������=ǰ��������ϡ���������+ǰ���η�Ӧ���Ľ���������-��Ӧ�ų�������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��2009?��ƽ��һģ����ѧʵ��С���ͬѧ��һ������Ca��OH��2��Һ��Na2CO3��Һ��ϣ���ַ�Ӧ����ˣ��õ���ɫ��Һ�����Ƕ���Һ�ijɷֽ���̽����
������⣺��Һ����ܺ�����Щ�����أ�
��������裺��ͬѧ������ܺ���NaOH��Ca��OH��2����ͬѧ������ܺ���NaOH��Na2CO3��Ca��OH��2
��ͬѧ�������ǡ����ȫ��Ӧֻ��NaOH����ͬѧ������ܺ��� ��1��______��
ʵ����֤����2������Լ�ͬѧ�IJ��룬���һ����ʵ�����֤����
��˼�����ۣ���3������Ϊ�ס��ҡ���ͬѧ�IJ��붼��������λͬѧ�����Ǵ���ģ������ԭ����______������Һ�к����������ʣ������ʵĿ�������У�д��ѧʽ��______��
���ʼ��𣺣�4����ѧС��ͬѧѡ�������ֲ�ͬ�����Լ�����ʵ�飬����ͬѧ���룬���ó�����--̼���ƴ��ڣ��뽫����Ļ�ѧ����ʽ�����±��У�
������⣺��Һ����ܺ�����Щ�����أ�
��������裺��ͬѧ������ܺ���NaOH��Ca��OH��2����ͬѧ������ܺ���NaOH��Na2CO3��Ca��OH��2
��ͬѧ�������ǡ����ȫ��Ӧֻ��NaOH����ͬѧ������ܺ��� ��1��______��
ʵ����֤����2������Լ�ͬѧ�IJ��룬���һ����ʵ�����֤����
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ͬѧ�IJ������ |
���ʼ��𣺣�4����ѧС��ͬѧѡ�������ֲ�ͬ�����Լ�����ʵ�飬����ͬѧ���룬���ó�����--̼���ƴ��ڣ��뽫����Ļ�ѧ����ʽ�����±��У�
| ��ѧ����ʽ | |
| �Լ�1 | |
| �Լ�2 |


