��Ŀ����
����Ŀ����֪��������Ҫ�ɷ��ǵ�����������
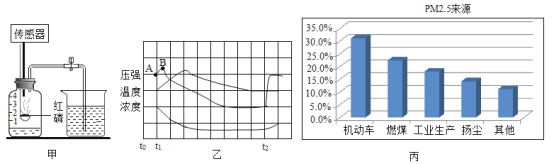
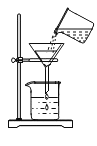
ij����С������˲ⶨ����������������ʵ�飬ʵ��װ����ͼ��ʾ��
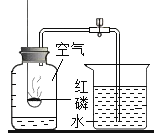
ʵ�鲽���У�������ֹˮ�мн���Ƥ�ܣ�
�ڵ�ȼȼ�ճ���ĺ��ף�
�۽�ȼ�ճײ�����ƿ�����������ӣ�
��ȼ����Ϻ�ֹˮ�С������ʵ��ش��������⣺
��1����ʵ���У������������Ŀ����___________������ȼ�յ����ֱ���ʽΪ��_______��
��2����ȼ�ճ��еĺ���ȼ�������ƿ�У��ܹ۲쵽��ʵ��������_________����ȼ��ֹͣ������ƿ��ȴ�����º�ֹˮ�У��۲쵽�ձ��е�ˮ���뼯��ƿ���ɴ˿�֪����Լռ�����������__________��
��3������������̿�ۣ���ʵ���ܷ�ɹ���_____����������������������������____��
��4����ȥ��Ƥ����Ѹ�ٽ�һ��ȼ�ŵ�ľ������ƿ�У��ῴ��_____��Ȼ�������ʯ��ˮ��������Ƥ�����۲쵽_________��
��5����������˵����������Ҫ����֧��ȼ�յ�______�����ʽ��ȶ���______��ɡ�
��6��ijͬѧ��ʵ����з�˼������˸Ľ���������ͼ��ʾ��������Ϊ�Ľ�����ŵ��ǣ�___________��
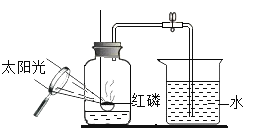
���𰸡��ú���ȼ��������ƿ�ڵ����� ����+����![]() ���������� ���������İ��̣��ų�����
���������� ���������İ��̣��ų����� ![]() ���� ̼��ȼ�����ɵ��Ƕ�����̼���壬����ƿ�ڵ�ѹǿ�����Ա仯��ˮ�������ƿ����ƿ ľ��Ϩ�� ����ʯ��ˮ�ޱ仯 ���� ���� װ��ʼ���ܱգ�����û���ݳ���ʵ������ȷ
���� ̼��ȼ�����ɵ��Ƕ�����̼���壬����ƿ�ڵ�ѹǿ�����Ա仯��ˮ�������ƿ����ƿ ľ��Ϩ�� ����ʯ��ˮ�ޱ仯 ���� ���� װ��ʼ���ܱգ�����û���ݳ���ʵ������ȷ
��������
��1����ʵ���к����Թ�����Ŀ�����ú�����ȫ����ƿ�е�����������ȼ���������������ף����ֱ���ʽΪ������+����![]() ���������ף�
���������ף�
��2������ȼ�յ������Dz��������İ��̣��ų���������ȼ��ֹͣ������ƿ��ȴ�����º�ֹˮ�У��۲쵽������ƿ��ˮԼռ���ƿ�ݻ���![]() ���ɴ˿�֪����Լռ�����������
���ɴ˿�֪����Լռ�����������![]() ��
��
��3������������̿�ۣ�ʵ�鲻�ܻ�óɹ��������ǣ�̼��ȼ�ղ����Ƕ�����̼���壬����ƿ����ѹ�����Ա仯��ˮ������뼯��ƿ��
��4������ȼ�պ��ƿ��ʣ�����Ҫ�ǵ�����������֧��ȼ�գ���˰�ȥ��Ƥ����Ѹ�ٽ�һ��ȼ�ŵ�ľ������ƿ�У��ῴ��ȼ�ŵ�ľ��Ϩ�𣻹��ƿ�ж�����̼�������٣���˵������ʯ��ˮ��������Ƥ���������ʯ��ˮ�ޱ仯��
��5����������˵����������Ҫ����֧��ȼ�յ����������ʽ��ȶ��ĵ�����ɣ�
��6��װ�øĽ�����ŵ㣺װ��ʼ���ܱգ�����û���ݳ���ʵ������ȷ��

����Ŀ��Ϊ�ⶨþ������þ����������þԪ�ص������������ȳ�ȡ�����10g������һ�ɾ����ձ��У�Ȼ��ȡһ����������������ϡ����100g��ƽ������μ������У������ʵ�������������±���
��1�� | ��2�� | ��3�� | ��4�� | ��5�� | |
����ϡ���������/g | 20 | 20 | 20 | 20 | 20 |
���������������/g | 0.12 | x | 0.36 | 0.4 | y |
��l���ϱ���x=_______��y=_______��
��2������ϡ�����������������Ϊ_______������������ȷ��0.1%��
��3������������þԪ�ص���������Ϊ����_______�����ڣ�3����д��������̣���������ȷ��0.1%��
����Ŀ��ij�ܱ�������ԭ��X��Y��������������̼��������,��һ�������³�ַ�Ӧ,��÷�Ӧǰ������ʵ��������±������ݱ�����Ϣ,�ж�����˵����ȷ���ǣ� ��
���� | X | Y | O2 | CO2 | H2O |
��Ӧǰ����/g | 16 | 15 | 70 | 1 | 0 |
��Ӧ������/g | 0 | 15 | ���� | 45 | 36 |
A.X��һ��ֻ��̼��������Ԫ��
B.�÷�Ӧһ��Ϊ�û���Ӧ
C.Yһ��Ϊ�÷�Ӧ�Ĵ���
D.��Ӧ��CO2��H2O��������һ��Ϊ45:36.