��Ŀ����
 ijУ��ѧ�о���ѧϰС���ͬѧ��ѧϰ�ˡ�CO�����ʡ���������ͼ��ʾ��̽��ʵ�飮ʵ�鷢��CO��CuO���Ⱥ��ɫ��ĩ��ɺ�ɫ��ĩ����������С����о����̲�����������
ijУ��ѧ�о���ѧϰС���ͬѧ��ѧϰ�ˡ�CO�����ʡ���������ͼ��ʾ��̽��ʵ�飮ʵ�鷢��CO��CuO���Ⱥ��ɫ��ĩ��ɺ�ɫ��ĩ����������С����о����̲�����������[�о�����]̽����ɫ��ĩ����Ҫ�ɷ�
[��������]
��1���й����ʵ���ɫ��CuO��ɫ��Cu2O��ɫ��Cu��ɫ��
��2��CuO��Cu2O���ܺ�ϡ���ᷢ����Ӧ����ѧ����ʽΪ��CuO+H2SO4�TCuSO4+H2O Cu2O+H2SO4�TCuSO4+Cu+H2O
[������ʵ��]
��1�����Ӳ�ʲ������ں�ɫ��ĩΪһ�����ʣ���������ijɷ֣�����Ƽ�ʵ��֤����IJ²⣮
| ���� | ��ʵ�鷽�� | ���� | CO��CuO��Ӧ�Ļ�ѧ����ʽ |
C
C
A����ӦǰCuO��ĩ������
B��Ӳ�ʲ������й������ʼ��ٵ�����
C��ͨ��CO������
D����Ӧ������������������
�������������֪����1��Cu��ĩ����ɫ���������Ӧ������CO��CuO���ȵķ�Ӧԭ����д��ѧ����ʽ��
Cu2O��ĩ����ɫ��Cu2O+H2SO4=CuSO4+Cu+H2O��������ɫ��Һ�ͺ�ɫ����ͭ����CO��CuO��������Cu2Oд����Ӧ����ʽ��
��2���������ں�ɫ��ĩΪ�������ʵĻ���Ҫȷ����ĩ���������ʵ�����������������Ը�����������м��㣮
Cu2O��ĩ����ɫ��Cu2O+H2SO4=CuSO4+Cu+H2O��������ɫ��Һ�ͺ�ɫ����ͭ����CO��CuO��������Cu2Oд����Ӧ����ʽ��
��2���������ں�ɫ��ĩΪ�������ʵĻ���Ҫȷ����ĩ���������ʵ�����������������Ը�����������м��㣮
����⣺��1���������⣺��ɫ��ĩΪһ�����ʣ����ɫ��ĩΪ��Cu��ĩ��Cu2O��ĩ��
����ͭ��������ͭ�����ʣ�������ϡ������м�����ʣ�����Ϊ��ɫ��ͭ������������������������ʣ�������������ͭ�����������������ɫ��Һ��
��2������Ϊ�������ݵ��ǣ�A����ӦǰCuO��ĩ��������B��Ӳ�ʲ������й������ʼ��ٵ�������C��һ����̼����ͨ��ͣ�ģ������ǹ����ģ�������ṩ�����������ڼ��㣻D����Ӧ�������������������ɸ��ݷ�Ӧǰ�����������������Ϸ�Ӧԭ���������
�ʴ�Ϊ����1��
��2��C��
����ͭ��������ͭ�����ʣ�������ϡ������м�����ʣ�����Ϊ��ɫ��ͭ������������������������ʣ�������������ͭ�����������������ɫ��Һ��
��2������Ϊ�������ݵ��ǣ�A����ӦǰCuO��ĩ��������B��Ӳ�ʲ������й������ʼ��ٵ�������C��һ����̼����ͨ��ͣ�ģ������ǹ����ģ�������ṩ�����������ڼ��㣻D����Ӧ�������������������ɸ��ݷ�Ӧǰ�����������������Ϸ�Ӧԭ���������
�ʴ�Ϊ����1��
| Cu | ȡ������ɫ��ĩ�������Թ��У������м���ϡ���� | ���������� | CuO+CO
| ||||
| Cu2O | ȡ������ɫ��ĩ�������Թ��У������м�������ϡ���� | ��Һ��Ϊ��ɫ�����к�ɫ���� | 2CuO+CO
|
������������Ҫ���黯ѧʵ�鷽������������ۣ�����һ����̼�Ļ�ѧ���ʣ��˽�ʵ��̽��������ɳɷ��Լ������ķ��������ʵ����������ʵ��������Ĺؼ���

��ϰ��ϵ�д�
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
�����Ŀ
Ϊ�˲ⶨijƷ��ʳ�ô�����̼���Ƶ�����������ijУ��ѧ�о���ѧϰС���̽���������£�
[�������]��Ʒ��̼���Ƶ����������Ƕ��٣�
[֪ʶ��]
ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
[��Ʒ�����ʵ��]
����ͬѧ����ȡ12.00��Ʒ����ˮ�����Һ������Һ�м����������ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g��
����ͬѧ����ȡ12.00��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���4.4g������̼��
[�������]
������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ������� ��̼���Ƶ����������� ������������ȷ��0.1%��
[������˼]
��1�������С��ͬѧ��Ϊ��Ҫ���̼���Ƶ�������Ҳ����ʹ���������ʯ��ˮ�������ͬ���������� ����һ�־������ʵĻ�ѧʽ������Һ����Ʒ��Ӧ��ͨ���ⶨ������ʵ������������йؼ��㼴�ɣ�
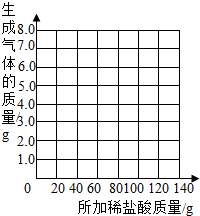
��2�������С��ͬѧ��Ϊ������ϡ�����������������Ҳ�������ȡ13.5g��Ʒ�����ձ��У�ÿ�μ���20gϡ���ᣨ������ˮ���Ȼ����ݳ������þ���������������¼ʵ���������£�
��������a= g��b= g�����������±ߵ�����ֽ�ϻ����������������������ϡ����������ϵ�����ߣ�
[�������]��Ʒ��̼���Ƶ����������Ƕ��٣�
[֪ʶ��]
ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
[��Ʒ�����ʵ��]
����ͬѧ����ȡ12.00��Ʒ����ˮ�����Һ������Һ�м����������ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g��
����ͬѧ����ȡ12.00��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���4.4g������̼��
[�������]
������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ�������
[������˼]
��1�������С��ͬѧ��Ϊ��Ҫ���̼���Ƶ�������Ҳ����ʹ���������ʯ��ˮ�������ͬ����������
��2�������С��ͬѧ��Ϊ������ϡ�����������������Ҳ�������ȡ13.5g��Ʒ�����ձ��У�ÿ�μ���20gϡ���ᣨ������ˮ���Ȼ����ݳ������þ���������������¼ʵ���������£�
| ��������Ĵ��� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| �ձ�����������������/g | 78.9 | 97.8 | 116.7 | 135.60 | 155.05 | 175.05 | 195.05 |
| �������������/g | 1.1 | 2.2 | a | 4.4 | 4.95 | b | -- |

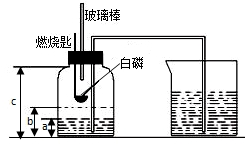 ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�
ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�