��Ŀ����
��6�֣��Ȼ�����Һ����ͭ��Ӧ���ɿ����Ե��Ȼ�ͭ���Ȼ���������ҵ�ϳ��ô�ԭ������ӡˢ��·�塣������ӡˢ��·�����������Һ�Ĵ������̣�
��ش��������⣺
��1������ڢ���ʵ������������� �������ʵ�����ǰ������������Ӧ��2FeCl3+Fe = 3FeCl2�� ��
��2��ij����ijɷ��� ��
��3�������������ϡ����ı�־�� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��4���������п���ѭ�����������õ������� ��
��1������ Fe+CuCl2=FeCl2+Cu
��2������ͭ(��Fe��Cu��
��3�����ٲ������� �� Fe+2HCl=FeCl2+H2 ��
��4��Cu��FeCl2
����

��8�֣�ij��ȤС���ʳ�ô����ʳ��С�մ����ַ�ĩ��������̽����
[��������]
| ���� | ʳ�ô��� | ʳ��С�մ� |
| ��Ҫ�ɷ� | Na2CO3 | NaHCO3 |
| ����� | ˮ��Һ�Լ��� | ˮ��Һ�Լ��� |
| ���ȶ��� | ���Ȳ��ֽ� | 270��ʱ��ȫ�ֽ�Ϊ̼���ơ�������̼��ˮ |
��1��̽������ˮ��Һ����ԵIJ���
С���ֱ����Ũ�ȵ�������Һ�е����̪��Һ���������߶��� ɫ����ʳ�ô�����Һ����ɫ����ɴ��Ʋ���� ��Һ���Ը�ǿ��С����ΪҪ�Ƚ�������Һ�ļ���ǿ�����ⶨ������ ��
��2����֤���ַ�ĩ���ȶ���
����ʦ��ָ���£���С�鰴��ͼ1װ�ý���ʵ��������۲쵽 ����ס����ҡ����ձ��г���ʯ��ˮ����ǣ��Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��װ������������ ��

С������ʵ��ʱ����ʵ��������һƿ��ǩ�������ɫҺ�壬��ͼ��ʾ��С���Ļ�ѧ��ȤС��Դ˲��������ʡ�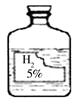
��������⡿��ƿ��ɫҺ����ʲô��?
���������롿���ݳ�����ѧ�Ļ�ѧ֪ʶ����Һ��ֻ����
����������Һ��̼�ᡢϡ���������ˮ�е�һ�֡�
�����۽����� ������ۺ�һ����Ϊ
��1��������������ˮ�������� ��
��2����������̼�ᣬ������̼��ȶ����ֽ⡣
��ʵ����֤��
Ϊȷ����Һ��ɷ֣�ͬѧ�Ǽ�������������ʵ��̽����
| ʵ����� | ʵ������ | ʵ����� |
| ����һ�� ȡ��Һ���������Թ��У������м������� ��ĩ | û�����ݲ����� | ��Һ�岻�ǹ���������Һ�� |
| ������� ȡ��Һ���������Թ��У������еμ������Ȼ�����Һ�� | | ��Һ����ϡ���ᡣ |
����˼������
��1�������Ϊ��ǩ�����ԭ������� ��
��2�������Һ���ǹ���������Һ�����û�ѧ����ʽ��ʾ����һ���������Ļ�ѧ��Ӧ�� ��
ijͬѧ����ͼװ����֤ij���������һ������ˮ������������̼��һ����̼�������塣
|
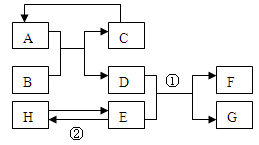
 ��1����ͬѧ��֤��������һ�����ڡ�ʵ�����ʱ����Ҫ��������������������⼸������������˳��Ӧ�ǣ���������( ) ��( ) ��( )�� E �� D��( ) ��( )����A��B��C��E�����ظ�ʹ�ã���
��1����ͬѧ��֤��������һ�����ڡ�ʵ�����ʱ����Ҫ��������������������⼸������������˳��Ӧ�ǣ���������( ) ��( ) ��( )�� E �� D��( ) ��( )����A��B��C��E�����ظ�ʹ�ã�����2���������ͨ��Aװ�÷�����Ӧ�Ļ�ѧ����ʽΪ______________________________
ij��ѧ��ȤС����ʵ����������ͼ��ʾ��A��B����ʵ�飮
��1��Bʵ���з�����Ӧ�Ļ�ѧ����ʽΪ�� ����
��2��A��B����ʵ�������С��ͬѧ����֧�Թ��е�����ͬʱ����һ�������ձ��У���ͼC��ʾ������Ϻ۲쵽�ձ�������ɫ������ͬѧ�ǽ��ձ��ڵ����ʹ��ˣ���������Һ�����ʵijɷֽ���̽����
�����롿
С�����룺��Һ�е�������Na2SO4��CuSO4��
С����룺��Һ�е�������Na2SO4��NaOH��
СӢ���룺��Һ�е�������Na2SO4��CuSO4��H2SO4��
��IJ��룺��Һ�е����ʻ��������� ����
�����ۡ�����Ϊ�� ���IJ���һ���������������ҺΪ��ɫ�������ų��� ���IJ��룬�������� ����
��ʵ�顿��С��IJ�����ȷ��������±��ķ�����
| ʵ�鲽�� | Ԥ���ʵ������ | ʵ����� |
| ȡ������Һ���Թ��У������� �� | �� �� | С��IJ�����ȷ |