题目内容
某课外活动小组加热氧化铜和炭粉(过量)的混合物,得到的铜粉中含有炭粉,他们用下图所示的装置,对获得的铜粉样品进行实验分析.以测定样品中铜的质量分数.(图中铁架台等装置已略去.)已知,过氧化氢在二氧化锰的催化作用下分解产生氧气.请你帮他们完成实验报告.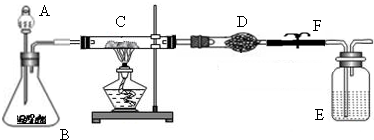
(1)实验过程:
| 编号 | 实验步骤 | 实验现象 | 有关化学方程式 |
| a | 连接好仪器后,打开止水夹F.检查 气密性(观察).在C中加入样品粉末 Wg,在D中装入药品后称量为m1g |
||
| b | 打开A的活塞,慢慢滴加溶液 | ||
| c | 对C进行加热当C中药品充分反应 后,关闭A的活塞、关F,停止加热 |
||
| d | 冷却后,称得D的质量为m2g |
样品中铜的质量分数=
(3)问题和讨论:
实验完成后,老师评议说:按上述实验设计,即使C中反应完全,D中吸收完全,也不会得出正确的结果.经时论,有同学提出在B与C之间加入一个装置.再次实验后.得到了较正确的结果,那么,原来实验所测得的铜的质量分数偏小的原因可能是在B与C之间加入的装置可以是
分析:(1)a检验气密性时,是对装置B进行微热,观察装置E中的现象.
b、打开A的活塞,慢慢滴加溶液将生成气体,发生反应2H2O2
2H2O+O2↑.
c、对C加热,发生反应2Cu+O2
=2CuO C+O2
CO2↑,根据反应进行现象叙述.
(2)根据质量守恒定律,可以根据D装置前后的质量变化求出二氧化碳的质量,根据二氧化碳中碳元素的质量分数求出碳元素的质量,进而求出铜的质量和质量分数.(3)根据实验目的和反应过程中进入装置D中的气体进行分析.
b、打开A的活塞,慢慢滴加溶液将生成气体,发生反应2H2O2
| ||
c、对C加热,发生反应2Cu+O2
| ||
| ||
(2)根据质量守恒定律,可以根据D装置前后的质量变化求出二氧化碳的质量,根据二氧化碳中碳元素的质量分数求出碳元素的质量,进而求出铜的质量和质量分数.(3)根据实验目的和反应过程中进入装置D中的气体进行分析.
解答:解:(1)a:检验气密性,首先微热装置B时,装置内压强增大,气体逸出,E中有气泡冒出,停止微热后玻璃管内形成一段液柱;
b:滴加过氧化氢溶液,发生反应2H2O2
2H2O+O2↑,所以再B、E中有气泡冒出,
C:加热装置发生反应2Cu+O2
=2CuO C+O2
CO2↑
C中的红褐色铜粉末变成黑色的氧化铜;
故答案为:
(2)根据质量守恒定律,反应生成的二氧化碳的质量为装置D反应前后的质量之差,为(m2-m1)g
同理混合物中碳的质量即为二氧化碳中碳的质量,即(m2-m1)×
所以铜的质量为w-(m2-m1)×
则铜的质量分数为:
×100%
故答案为:
×100%
(3)因为装置B中氧气逸出时要携带水蒸气,水蒸气通过C可以被装置D的碱石灰吸收,而影响实验结果.所以应在B和C之间加一个洗气瓶,以除去氧气中的水蒸气.
故答案为:水蒸气通过装置C被装置D中的碱石灰吸收
洗气瓶; 浓硫酸(或干燥管; 碱石灰)
b:滴加过氧化氢溶液,发生反应2H2O2
| ||
C:加热装置发生反应2Cu+O2
| ||
| ||
C中的红褐色铜粉末变成黑色的氧化铜;
故答案为:
| 编号 | 实验步骤 | 实验现象 | 有关化学方程式 | ||||||||
| a | 连接好仪器后,打开止水夹F.检查 气密性(观察).在C中加入样品粉末 Wg,在D中装入药品后称量为m1g |
微热装置B时E中有气泡冒出,停止加热后玻璃管内形成一段液柱 | |||||||||
| b | 打开A的活塞,慢慢滴加溶液 | B、E中有气泡冒出 | 2H2O2
| ||||||||
| c | 对C进行加热当C中药品充分反应 后,关闭A的活塞、关F,停止加热 |
C中的红褐色粉末变成黑色 | 2Cu+O2
C+O2
| ||||||||
| d | 冷却后,称得D的质量为m2g |
同理混合物中碳的质量即为二氧化碳中碳的质量,即(m2-m1)×
| 12 |
| 44 |
所以铜的质量为w-(m2-m1)×
| 12 |
| 44 |
则铜的质量分数为:
w-(m2-m1)×
| ||
| w |
故答案为:
w-(m2-m1)×
| ||
| w |
(3)因为装置B中氧气逸出时要携带水蒸气,水蒸气通过C可以被装置D的碱石灰吸收,而影响实验结果.所以应在B和C之间加一个洗气瓶,以除去氧气中的水蒸气.
故答案为:水蒸气通过装置C被装置D中的碱石灰吸收
洗气瓶; 浓硫酸(或干燥管; 碱石灰)
点评:在设计干燥装置时要注意:一种是洗气瓶,药品是液体干燥剂,一种是干燥管,药品是固体干燥剂.

练习册系列答案
相关题目
某课外活动小组的同学将过量的炭粉和16克氧化铜均匀混合,用下图所示装置进行实验.图中铁架台等装置已略去.请回答有关问题:
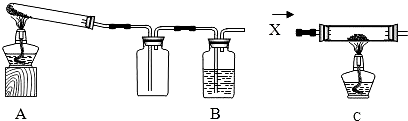
(1)同学们想通过测定消耗碳、氧元素的质量和生成二氧化碳的质量,分析氧化铜和炭粉反应产生的气体除CO2外是否还有其它产物.反应一段时间后停止加热,冷却到室温.反应前、后测得的数据如下:
分析数据发现,反应中消耗碳和氧元素的质量 (填“大于”、“小于”或“等于”)生成二氧化碳的质量.下列4项中跟这一结果有关的有哪几项? .(填写字母)
A.装置中还有一部分CO2未被石灰水溶液吸收
B.氧化铜和炭粉反应产生的气体除CO2外还有CO
C.氧化铜和炭粉没有完全反应
D.该反应不符合质量守恒定律
(2)有同学认为用图示装置C代替上面的装置A,加热前先通一会气体X,停止加热后再通一会该气体,这样可使实验测得的数据更能准确的说明问题.你认为在O2、N2和H2三种气体中,X应选择哪一种气体? .
(3)实验中最多能得到铜多少克?(写出计算过程)

(1)同学们想通过测定消耗碳、氧元素的质量和生成二氧化碳的质量,分析氧化铜和炭粉反应产生的气体除CO2外是否还有其它产物.反应一段时间后停止加热,冷却到室温.反应前、后测得的数据如下:
| 装置 | 反应前 | 反应后 |
| A | 试管的质量38.2 克 氧化铜和炭粉混合物的质量20.0克 |
试管和固体物质的质量56.8 克 |
| B | 反应后瓶内石灰水比反应前增重1.1 克 | |
A.装置中还有一部分CO2未被石灰水溶液吸收
B.氧化铜和炭粉反应产生的气体除CO2外还有CO
C.氧化铜和炭粉没有完全反应
D.该反应不符合质量守恒定律
(2)有同学认为用图示装置C代替上面的装置A,加热前先通一会气体X,停止加热后再通一会该气体,这样可使实验测得的数据更能准确的说明问题.你认为在O2、N2和H2三种气体中,X应选择哪一种气体?
(3)实验中最多能得到铜多少克?(写出计算过程)
| 某课外活动小组的同学将过量的炭粉和16克氧化铜均匀混合,用下图所示装置进行实验。图中铁架台等装置已略去。请回答有关问题: | ||
 | ||
| (1)同学们想通过测定消耗碳、氧元素的质量和生成二氧化碳的质量,分析氧化铜和炭粉反应产生的气体除CO2外是否还有其它产物。反应一段时间后停止加热,冷却到室温。反应前、后测得的数据如下: | ||
 | ||
| 分析数据发现,反应中消耗碳和氧元素的质量 (填“大于”、“小于”或“等于”)生成二氧化碳的质量。下列4项中跟这一结果有关的有哪几项? 。(填写字母) A.装置中还有一部分CO2未被石灰水溶液吸收 B.氧化铜和炭粉反应产生的气体除CO2外还有CO C.氧化铜和炭粉没有完全反应 D.该反应不符合质量守恒定律 | ||
|
某课外活动小组的同学将过量的炭粉和16克氧化铜均匀混合,用下图所示装置
进行实验.图中铁架台等装置已略去.请回答有关问题:
(1)同学们想通过测定消耗碳、氧元素的质量和生成二氧化碳的质量,分析氧化铜和炭粉反应产生的气体除CO2外是否还有其它产物.反应一段时间后停止加热,冷却到室温.反应前、后测得的数据如下:
分析数据发现,反应中消耗碳和氧元素的质量______(填“大于”、“小于”或“等于”)生成二氧化碳的质量.下列4项中跟这一结果有关的有哪几项?______.(填写字母)
A.装置中还有一部分CO2未被石灰水溶液吸收
B.氧化铜和炭粉反应产生的气体除CO2外还有CO
C.氧化铜和炭粉没有完全反应
D.该反应不符合质量守恒定律
(2)有同学认为用图示装置C代替上面的装置A,加热前先通一会气体X,停止加热后再通一会该气体,这样可使实验测得的数据更能准确的说明问题.你认为在O2、N2和H2三种气体中,X应选择哪一种气体?______.
(3)实验中最多能得到铜多少克?(写出计算过程)

进行实验.图中铁架台等装置已略去.请回答有关问题:
(1)同学们想通过测定消耗碳、氧元素的质量和生成二氧化碳的质量,分析氧化铜和炭粉反应产生的气体除CO2外是否还有其它产物.反应一段时间后停止加热,冷却到室温.反应前、后测得的数据如下:
| 装置 | 反应前 | 反应后 |
| A | 试管的质量38.2 克 氧化铜和炭粉混合物的质量20.0克 | 试管和固体物质的质量56.8 克 |
| B | 反应后瓶内石灰水比反应前增重1.1 克 | |
A.装置中还有一部分CO2未被石灰水溶液吸收
B.氧化铜和炭粉反应产生的气体除CO2外还有CO
C.氧化铜和炭粉没有完全反应
D.该反应不符合质量守恒定律
(2)有同学认为用图示装置C代替上面的装置A,加热前先通一会气体X,停止加热后再通一会该气体,这样可使实验测得的数据更能准确的说明问题.你认为在O2、N2和H2三种气体中,X应选择哪一种气体?______.
(3)实验中最多能得到铜多少克?(写出计算过程)
 某课外活动小组对红褐色铜粉(含炭)样品进行实验,所用装置如图所示(图中部分仪器装置已略去).
某课外活动小组对红褐色铜粉(含炭)样品进行实验,所用装置如图所示(图中部分仪器装置已略去). 某课外活动小组对红褐色铜粉(含炭)样品进行实验,所用装置如图所示(图中部分仪器装置已略去).
某课外活动小组对红褐色铜粉(含炭)样品进行实验,所用装置如图所示(图中部分仪器装置已略去).