��Ŀ����
����Ŀ��ˮ������֮Դ������֮���������౦�����Ȼ��Դ������ÿ���˶�Ҫ����ˮ������ˮ����Լˮ��
��1���ҹ��涨��������ˮ��ˮ�ʱ���ﵽ����ָ�꣺
a.���ó�����ɫ�� b.��������ζ�� c.Ӧ������
���С�ab��ָ�����ͨ������_____�ﵽ����c��ָ�����ͨ��_____�����ﵽ��
��2������ˮ�������㣬����Ҫԭ��������ˮ�м�������_____��
����Ԫ�� ��ˮ���� ����ԭ�� ��������
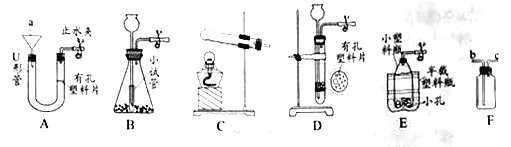
��3����ͼΪ���ˮ��װ�á�B���е�����Ϊ_____��
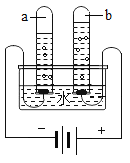
�÷�Ӧ�ķ���ʽΪ_____��
��4������ˮ����Ȫˮ���״ס��ƾ����dz�������ɫҺ�壬���а״�����ζ���ƾ�����������ζ��������Ϊ��ɫ��Դ����д���ƾ�ȼ�յĻ�ѧ����ʽ_____������������ɫҺ����ö��ַ������֣����磺����_____�����ݲ�������ĭ���������֡�
���𰸡�����̿ ���� �� ���� 2H2O![]() 2H2����O2�� C2H5OH��3O2
2H2����O2�� C2H5OH��3O2![]() 2CO2��3H2O ����ˮ
2CO2��3H2O ����ˮ
��������
��1���ҹ��涨��������ˮ��ˮ�ʱ���ﵽ����ָ�꣺
a.���ó�����ɫ�� b.��������ζ�� c.Ӧ������
���С�ab��ָ�����ͨ���������̿�ﵽ����Ϊ����̿������ɫ�غ���ζ�����ã���c��ָ�����ͨ�����˲�����ȥ�����
��2������ˮ�������㣬����Ҫԭ��������ˮ�м������������ӣ����ܹ������������
��3�����ˮ��װ�á�b�����ӵ�Դ����������������Ϊ������
���ˮ���������������ķ���ʽΪ��2H2O![]() 2H2����O2����
2H2����O2����
��4���ƾ�ȼ�ղ���������̼��ˮ�Ļ�ѧ����ʽC2H5OH��3O2![]() 2CO2��3H2O������������ɫҺ����ö��ַ������֣����磺�������ˮ����Ͻ�����������ĭ���������ˮ����֮�ǿ�Ȫˮ����
2CO2��3H2O������������ɫҺ����ö��ַ������֣����磺�������ˮ����Ͻ�����������ĭ���������ˮ����֮�ǿ�Ȫˮ����

����Ŀ���ܽ�����ߵ�����
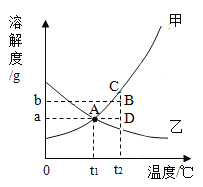
�ٱ�ʾ�ܽ�����¶ȱ仯���ƣ�����������ܽ�����¶����߶�������_____�����������ܽ�����¶����߶��仯������_____�������������ܽ�����¶����߶���С��_____��
��ȷ��ij�¶���ij���ʵ�_____��
�۽����ʾ�������ڸ�_____���ܽ��_____��
���ܽ��������һ�㣨��A�㣩��ʾt1������ʵ�һ��_____��Һ������Һ�����ʣ��ܼ�����Һ��������=_____��
���ܽ��������һ�㣨��B�㣩��ʾt2������ʵ�һ��_____��Һ������Һ�����ʣ��ܼ�����Һ��������=_____��
��
ʵ����� | A����Һ�ƶ� | B����Һ�ƶ� |
���� | _____ | _____ |
���� | _____ | _____ |
�����ܼ� | _____ | _____ |
������ | _____ | _____ |
��50gB���������Һ��ΪD����Һ���;����_____��
����Ŀ���������Ƕ����������ɽϴ�����Ҳ��������һ�棬��ijЩʳƷ��װ���ڳ����뻹ԭ����������˫�������Է�ֹʳƷ���ܣ�ij������ȤС����ʵ���ҷ�����һ������Ļ�ԭ�����ۣ����������ʣ����ʼȲ�����ˮҲ�������ᣩ������ȡ������Ʒ���ֱ��ϡ���ᷴӦ����ò������ݼ��£�������й���Ϣ�մ����⣮
ʵ����� | 1 | 3 | 4 | 5 | 6 |
ȡ��Ʒ������g�� | 31.0 | 31.0 | 31.0 | 31.0 | 31.0 |
ȡϡ����������g�� | 30.0 | 90.0 | 120.0 | 150.0 | 180.0 |
��������������g�� | 0 | a | 0.3 | 0.5 | 0.6 |
��1��ʳƷ���ڷ��롰˫��������Ϊ������ �������۶�������������˫�������������� ��
��2��a����ֵΪ ��
��3����4��ʵ�飬���õ���Һ�����ʵĻ�ѧʽΪ ��
��4����ʽ�������Ʒ�е�����������������������0.1%����
����Ŀ��ij����С��Ϊ�ⶨ����ʯ��ʯ�к�̼��Ƶ�����������ȡ����һЩ��ʯ����ȡϡ����200g��ƽ���ֳ�4�ݣ�����ʵ�飬������£�
ʵ�� | 1 | 2 | 3 | 4 |
������Ʒ������/g | 5 | 10 | 15 | 20 |
����CO2������/g | 1.54 | 3.08 | 4.4 | m |
��1���ļ��Ӧ��������ʣ��_______��
��2��m=______g��
��3���Լ�������ʯ��ʯ����̼��Ƶ���������_________��