��Ŀ����
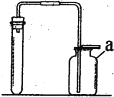
����Ŀ���������۲��������ľ̿��ԭ����ͭ������ʾʵ�飬������ͼ���ش����⣺

(1)д����ľ̿��ԭ����ͭ�Ļ�ѧ����ʽ��______________��
(2)��Ӧ����һ��ʱ���Ϊ�˽�һ��̽���Թ���ʣ��������ɣ����Թ���ȴ�����º�ȡʣ��������ձ��У��ټ������ϡ���ᣬ��ֽ������ˡ�
������ֽ�����к�ɫ����ɫ���壬����ҺΪ��ɫ������Һ�е�������_______���Թ��е�ʣ�����Ϊ_______��
������ֽ�����к�ɫ����ɫ���壬����Һ����ɫ����ʱ��Һ�е�������_______��
���𰸡� C��2CuO![]() 2Cu��CO2�� ���ᣨ��H2SO4�� ̼��ͭ����C��Cu�� ����ͭ�����ᣨ��CuSO4��H2SO4��
2Cu��CO2�� ���ᣨ��H2SO4�� ̼��ͭ����C��Cu�� ����ͭ�����ᣨ��CuSO4��H2SO4��
������������1��ľ̿������ͭ�ڸ����·�Ӧ����ͭ�Ͷ�����̼����ѧ����ʽΪ��C+2CuO 2Cu+CO2����
��2����ͭ��̼��ϡ�����Ӧ������ͭ��ϡ���ᷴӦ����ֽ�����к�ɫ����ɫ���壬��ҺΪ��ɫ��˵��ʣ�������û������ͭ����ɫ����һ����ľ̿������ϡ����û�вμӷ�Ӧ������Һ������Ϊ���ᣬ�Թ��е�ʣ�����Ϊľ̿��
������ֽ���к�ɫ����ɫ���壬����Һ����ɫ��˵������ͭ��ľ̿��ʣ�࣬��ʱ��Һ�е�����һ����������������ͭ��Ӧ���ɵ�����ͭ���������ǹ����ģ��������ʻ������ᣮ
�ʴ�Ϊ��
��1��C+2CuO 2Cu+CO2����
��2����H2SO4ľ̿��Cu����CuSO4��H2SO4

����Ŀ�����ǵ��ճ������벻���������߿Ƽ��²��ϵĿ�����Ӧ��Ҳ��Ҫ������
(1)�ؿ��к�����ߵĽ���Ԫ����__________��
(2)����ͼʾ����Ӧ��ʵ���ƶϣ��������е�����������___________________��

(3)�ճ�ʹ�õĽ������϶������ںϽ𣮱����г���һЩ�����Ͻ����Ҫ�ɷֺ����ܣ�
�Ͻ� | �Ͻ����Ҫ���� | ��Ҫ�ɷּ������������� |
���� | �۵�183�� | �����۵�232�棻Ǧ���۵�327�� |
Ӳ�� | ǿ�Ⱥ�Ӳ�Ⱥ� | ����ͭ��þ�ȣ�Ӳ��С������ |
����� | ����ʴ�Ժ� | �����������ȣ���������ʴ���ܲ��粻��� |
���ϱ��ƶϣ�����ɺϽ�Ĵ�������ȣ��Ͻ���ŵ�һ����________________��
��ǿ�ȸ��͢�Ӳ�ȸ��� ���۵���� �ܿ���ʴ�Ը���
(4)����ÿ��Ҫ�ӽ���������Դ����ȡ�����ڶּƵĽ�����������ѧ��ѧ֪ʶ����Ҫ ��д���������ɽ����Ļ�ѧ����ʽ��
��___________________________________________________(�û���Ӧ)��
��___________________________________________________(�ֽⷴӦ) ��
(5)2008����˻����˶���������ʹ���˴����ĸ�����������_____________ֱ�ӽӴ��������������ʧ���ڸ�������Ϳ�͡�ˢ��ȣ����ܷ�ֹ�������⣮