МвДїДЪИЭ
ЎѕМвДїЎїВнУўН¬С§ИЎДіµШКЇ»ТКЇСщЖ·12 gЅшРРІв¶ЁКµСйЈ¬ПЦЅ«100 gПЎСОЛб·ЦОеґОјУИлКЇ»ТКЇСщЖ·ЦР(ФУЦКІ»ИЬУЪЛ®ТІІ»ІОУл·ґУ¦)Ј¬ід·Ц·ґУ¦єуІвµГЙъіЙЖшМеµДЧЬЦКБїИз±нЛщКѕЈє
µЪ1ґО | µЪ2ґО | µЪ3ґО | µЪ4ґО | µЪ5ґО | |
јУИлПЎСОЛбµДЦКБї/g | 20 | 20 | 20 | 20 | 20 |
ЙъіЙЖшМеµДЧЬЦКБї/g | 1.1 | 2.2 | m | 4.4 | 4.4 |
КФЗуЈє
(1)mµДЦµОЄ________gЎЈ
(2)КЇ»ТКЇСщЖ·ЦРМјЛбёЖµДЦКБї·ЦКэ______ЎЈ(РґіцјЖЛг№эіМЈ¬јЖЛгЅб№ыѕ«И·ЦБ0.1%)
Ўѕґр°ёЎї 3.3 83.3%
ЎѕЅвОцЎї(1)·ЦОеґОјУИлСОЛбєуЈ¬З°БЅґОЈ¬ГїґО¶јФцјУ1.1g¶юСх»ЇМјЈ¬µЪЛДЎўОе¶јЙъіЙ4.4g¶юСх»ЇМјЈ¬ЛµГчµЪИэґОГ»УРНкИ«·ґУ¦Ј¬ТІФцјУ1.1gЈ¬ЛщТФmЦµКЗ3.3gЈ»(2)ЙиІОјУ·ґУ¦µДМјЛбёЖЦКБїОЄx
CaCO3+2HCl=CaCl2+H2O+CO2Ўь
100 44
x 4.4g
![]() Ј¬x=10gЈ¬КЇ»ТКЇСщЖ·ЦРМјЛбёЖµДЦКБї·ЦКэОЄ
Ј¬x=10gЈ¬КЇ»ТКЇСщЖ·ЦРМјЛбёЖµДЦКБї·ЦКэОЄ![]() ЎЈ
ЎЈ
ґрЈє(1) mµДЦµОЄ3.3gЈ»(2) КЇ»ТКЇСщЖ·ЦРМјЛбёЖµДЦКБї·ЦКэОЄ83.3%ЎЈ

 ГыРЈїОМГПµБРґр°ё
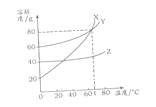
ГыРЈїОМГПµБРґр°ёЎѕМвДїЎїВИ»ЇДЖєНБтЛбГѕµДИЬЅв¶ИЗъПЯУлИфёЙОВ¶ИПВµДИЬЅв¶И±нИзПВЈє
ОВ¶И/Ўж | 20 | 30 | 40 | 60 | 80 | 90 | 100 | |
ИЬЅв¶И (g/100GH20) | NaCl | 36.0 | 36.3 | 36.6 | 37.3 | 38.4 | 39.0 | 39.8 |
MgSO4 | 33.7 | 38.9 | 44.5 | 54.6 | 55.8 | 52.9 | 50.4 | |

Зл»ШґрПВБРОКМвЈє
(1)80ЎжК±Ј¬БтЛбГѕµДИЬЅв¶ИОЄ__________Ј¬ИЬЅв¶ИЗъПЯјЧЛщґъ±нµДОпЦККЗ__________ЎЈaµг¶ФУ¦µДОВ¶Иt1·¶О§КЗ____________________ЎЈ
(2)t2ЎжК±УРє¬УРЅП¶аNaCl µДMgSO4ИЬТєЈ¬ОЄБЛµГµЅґїѕ»µДMgSO4Ј¬їЙІЙУГµД·Ѕ·Ё__________
(3)40ЎжК±°С20g NaCl·ЕИл50gЛ®ЦРЈ¬РОіЙµДИЬТєЦРИЬЦКЦКБї·ЦКэОЄ__________(ѕ«И·µЅ0.1%)Ј¬ТЄК№t3Ўж±ҐєНµДMgSO4ИЬТєЦРИЬЦКЦКБї·ЦКэФцґуЈ¬їЙІЙУГµДґлК©КЗ__________ЎЈ