��Ŀ����
��7�֣�ʵ������һ����ĩ״���ʣ�������NaCl��Na2SO4��Na2CO3��FeCl3�е�һ�ֻ�����ɡ�Ϊ��ȷ��ԭ��ĩ�ijɷ֣�ijУ��ѧ��ȤС���ͬѧ������ͼ��ʾ����ʵ�飺
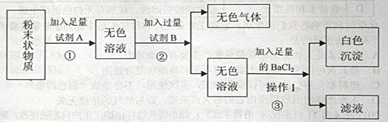
��ش��������⣺���ѧʽ��
��1����A����Է���������С���������AΪ ����ɫ������ ��
��2���÷�ĩ��һ������ ��������������� ��
��3��Ϊ�˲����ŶԵڢ۲�ʵ��������жϣ��Լ�B���ѡ�� ����д������۵Ļ�ѧ����ʽ�� ��

��ش��������⣺���ѧʽ��
��1����A����Է���������С���������AΪ ����ɫ������ ��
��2���÷�ĩ��һ������ ��������������� ��
��3��Ϊ�˲����ŶԵڢ۲�ʵ��������жϣ��Լ�B���ѡ�� ����д������۵Ļ�ѧ����ʽ�� ��
(ÿ��1�֣���7��) ��1��H2O CO2 ��2��FeCl3 ����
���������ݣ�1����AΪ��Է���������С����������ˮ����ˮ��õ�����ɫ��Һ���Ȼ�����ˮ��ҺΪ��ɫ������ȷ��û���Ȼ������ܹ��������������ֻ��̼���ƣ����Լ�BΪ���ᣬ����ɫ����Ϊ������̼�����ڼ���������ǹ����ģ����Եõ��İ�ɫ����Ϊ���ᱵ������ԭ������к��������ƣ�����IΪ���ˣ����˷�����ϣ����Ծݴ˴��⣮
�⣺��1��AΪ��Է���������С�����������֪��AΪˮ���������B��õ���ɫ���壬�������ܹ����������ֻ��̼���ƣ����Կ����жϺ���̼���ƣ���ô���ɵ���ɫ����Ϊ������̼��
��2�������Լ�B��õ�����ɫ��Һ�������Ȼ�����Һ�ʻ�ɫ�����Կ����ƶϸ÷�ĩ�в����Ȼ��������ڲ���I��õ��ǹ���Ҳ��Һ�����Կ����жϲ���IΪ���ˣ�
��3���������ӵ��Լ��Ȼ��������жϳ����ɵİ�ɫ����Ϊ���ᱵ������Ϊ�˷�ֹ��ʵ�����ĸ��ţ��Լ�B��ü���������ᣬ�ʲ�����������ƺ��Ȼ����ķ�Ӧ��
�ʴ�Ϊ����1��H2O�� CO2
��2��FeCl3������
��3��ϡ��� Na2SO4+BaCl2�T2NaCl+BaSO4��
����������Ϊ���������������ƶ��⣬��ɴ��⣬Ҫץס���������Ĺؼ������ݸ���������֮��ķ�Ӧ��������ͻ�ƿڣ������жϣ���ǻ�ѧ����ʽ��Na2SO4+BaCl2�T2NaCl+BaSO4����
�⣺��1��AΪ��Է���������С�����������֪��AΪˮ���������B��õ���ɫ���壬�������ܹ����������ֻ��̼���ƣ����Կ����жϺ���̼���ƣ���ô���ɵ���ɫ����Ϊ������̼��
��2�������Լ�B��õ�����ɫ��Һ�������Ȼ�����Һ�ʻ�ɫ�����Կ����ƶϸ÷�ĩ�в����Ȼ��������ڲ���I��õ��ǹ���Ҳ��Һ�����Կ����жϲ���IΪ���ˣ�
��3���������ӵ��Լ��Ȼ��������жϳ����ɵİ�ɫ����Ϊ���ᱵ������Ϊ�˷�ֹ��ʵ�����ĸ��ţ��Լ�B��ü���������ᣬ�ʲ�����������ƺ��Ȼ����ķ�Ӧ��
�ʴ�Ϊ����1��H2O�� CO2
��2��FeCl3������
��3��ϡ��� Na2SO4+BaCl2�T2NaCl+BaSO4��
����������Ϊ���������������ƶ��⣬��ɴ��⣬Ҫץס���������Ĺؼ������ݸ���������֮��ķ�Ӧ��������ͻ�ƿڣ������жϣ���ǻ�ѧ����ʽ��Na2SO4+BaCl2�T2NaCl+BaSO4����

��ϰ��ϵ�д�
 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ