��Ŀ����
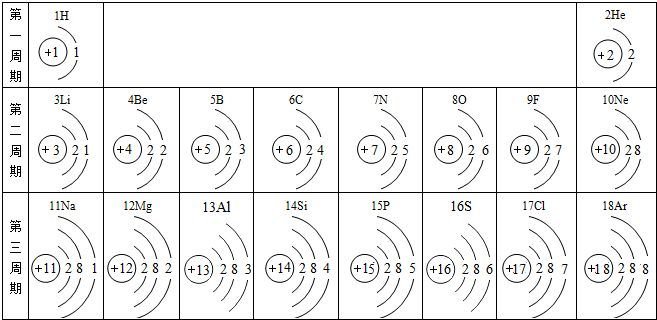
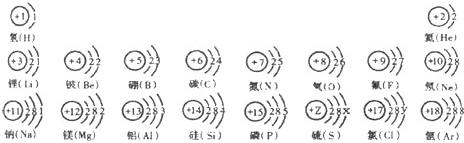
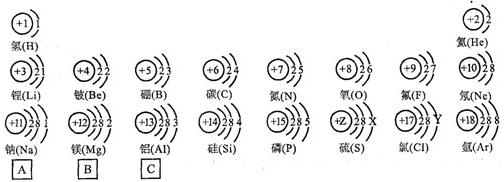
�����ݺ˵����Ϊ1---18��Ԫ��ԭ�ӽṹʾ��ͼ���ش��������⣺
| ��һ���� | 1H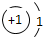 | 2He | ||||||
| �ڶ����� | 3Li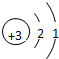 | 4Be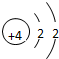 | 5B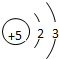 | 6C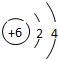 | 7N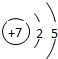 | 8O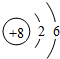 | 9F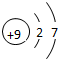 | 10Ne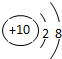 |
| �������� | 11Na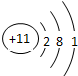 | 12Mg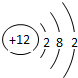 | 13Al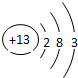 | 14Si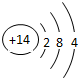 | 15P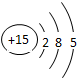 | 16S | 17Cl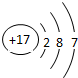 | 18Ar |
��2������Ԫ���������________������ţ�
Aԭ�Ӻ��ڵ������� B����������C���ԭ������Dԭ�Ӻ��ڵ�������
��3����ͼ�����о���һ���Ĺ��ɣ��磺ͬһ�����У�������Ԫ�صĺ˵�����������ӣ��㻹���ҵ���Щ���ɣ���д������һ��________��˵��һ�㣩��
�⣺��1������Ԫ�������ɣ�ͬһ�����У�������Ԫ�صĺ˵�����������ӣ���֪��Ԫ�صĺ˵���� Z=16����Ԫ�ص�����������Y=7��
����Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ�йأ�������������ͬ��Ԫ�ػ�ѧ�������ƣ�����Ԫ�ػ�ѧ�������Ƶ�Ԫ���Ƿ���F����
�ʴ�Ϊ��16��7������F����
��2�����ݲ�ͬ��Ԫ��֮��ı�����������������ͬ����ѡA��
��3������Ԫ�������ɵ�֪ʶ��ͼ���е���Ϣ�ɵã�ͬһ�ݺ��������������ͬ��
�ʴ�Ϊ��ͬһ�ݺ��������������ͬ��
��������1������Ԫ�������ɣ�ͬһ�����У�������Ԫ�صĺ˵�����������ӣ����н��
����Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ�йأ�������������ͬ��Ԫ�ػ�ѧ�������ƣ����н��
��2�����ݲ�ͬ��Ԫ��֮��ı�����������������ͬ�����н��
��3������Ԫ�������ɵ�֪ʶ��ͼ���е���Ϣ���н��
���������⿼��ѧ����Ԫ�����ڱ���Ԫ��������֪ʶ���������ڽ��������Ӧ�õ�������
����Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ�йأ�������������ͬ��Ԫ�ػ�ѧ�������ƣ�����Ԫ�ػ�ѧ�������Ƶ�Ԫ���Ƿ���F����
�ʴ�Ϊ��16��7������F����
��2�����ݲ�ͬ��Ԫ��֮��ı�����������������ͬ����ѡA��
��3������Ԫ�������ɵ�֪ʶ��ͼ���е���Ϣ�ɵã�ͬһ�ݺ��������������ͬ��
�ʴ�Ϊ��ͬһ�ݺ��������������ͬ��
��������1������Ԫ�������ɣ�ͬһ�����У�������Ԫ�صĺ˵�����������ӣ����н��
����Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ�йأ�������������ͬ��Ԫ�ػ�ѧ�������ƣ����н��
��2�����ݲ�ͬ��Ԫ��֮��ı�����������������ͬ�����н��
��3������Ԫ�������ɵ�֪ʶ��ͼ���е���Ϣ���н��
���������⿼��ѧ����Ԫ�����ڱ���Ԫ��������֪ʶ���������ڽ��������Ӧ�õ�������

��ϰ��ϵ�д�
�����Ŀ



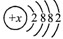 ��ʾ�����Ԫ�ص�ԭ������Ϊ
��ʾ�����Ԫ�ص�ԭ������Ϊ