��Ŀ����
����Ŀ����ˮ�к���NaCl��Na2CO3�����ʡ���ˮ���������ú�ˮ����ȼú������SO2��

��1����ˮ�����豸�У��ų��ĺ�ˮ�ʼ��Ե���_______������ĸ��ţ���
A����ˮ�� B�������� C. �����آ� D�������آ�
��2���������آ���ͨ�������������Ҫ��ѧ��Ӧ���£�
i. 2H2SO3 + O2 ==== 2H2SO4
ii. 2Na2SO3 + O2 ==== 2Na2SO4
��Ӧi��ii�л��ϼ����ߵ�Ԫ����_______��
��3�������آ��ŷŵĺ�ˮ�в���H2SO4��ԭ����_______���û�ѧ����ʽ���ͣ���
���𰸡� A S Na2CO3 + H2SO4 ===== Na2SO4 + CO2��+ H2O
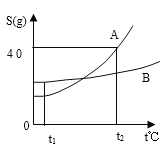
����������1��������ҺpH���
��2������Ԫ�ػ��ϼ۽��
��3������̼���������ᷴӦ���
�⣺��1����ͼ��֪��ˮ�ó���ĺ�ˮpH=8���ʺ�ˮ�����豸�У��ų��ĺ�ˮ�ʼ��Ե��Ǻ�ˮ�ã���ѡA��
��2����H2SO3��S��+4�ۣ���Na2SO4��H2SO4��S��+6�ۡ��ʷ�Ӧi��ii�л��ϼ����ߵ�Ԫ���ǣ�S��
��3�����ں�ˮ�к���̼���ƣ�̼���������ᷢ����Ӧ���������ơ�ˮ�Ͷ�����̼���������آ��ŷŵĺ�ˮ�в���H2SO4��ԭ����Na2CO3 + H2SO4 = Na2SO4 + CO2��+ H2O��