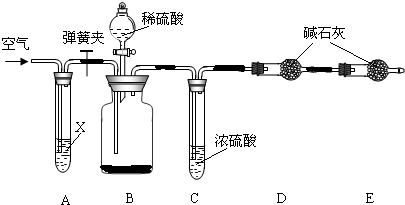
��Ŀ����
����IJ����Ǻ���һ�����һ�ѧ��ҵ��չˮƽ����Ҫָ�꣮������ʵ������������Ƽ����ԭ������Ҫ���̣�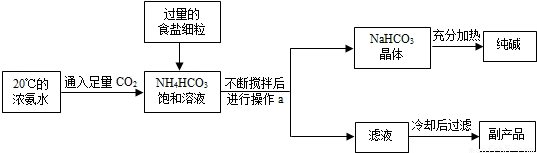
��ش��������⣺
��1����������______��
A���� B���� C���� D������
��2���ڢڲ��м������ĥϸʳ�ηۣ�ʳ��ĥϸ��Ŀ���ǣ�______������a��������______��
��3������ʳ�κ���д���������ֽⷴӦ�Ļ�ѧ����ʽ______��
��4�������Ƽ���ŵ�֮һ�����õĸ���Ʒ�Ȼ����һ�ֵ��ʣ���Ҫͨ��ʵ�������Ȼ�狀���һ�ֵ�������泥����õ��Լ���______��Һ����һ���ŵ��DZȽϻ�������Щ���ʿ���ѭ�����ã�����Ϊ�����������У�����ѭ�������õ�������______��
���𰸡���������1�����εĹ������жϣ�
��2���ӷ�Ӧ��ĽӴ�����Ϸ������Ӳ������������жϣ�
��3�����������غ㶨��д����ʽ��
��4���������Ӻ������ӷ�Ӧ���ɳ����жϣ���Ӧ����������Ϸ�����
����⣺��1��̼�������ɽ������Ӻ�������ӹ��ɵģ����Σ��ʴ�Ϊ��C
��2��ʳ��ĥϸ��Ŀ�ľ�������Ӵ������ʹʳ����NH4HCO3��ַ�Ӧ���ѹ����Һ�����IJ����ǹ��ˣ��ʴ�Ϊ������Ӵ������ʹʳ����NH4HCO3��ַ�Ӧ������
��3���Ȼ��ƺ�̼����立�Ӧ����̼�����ƺ��Ȼ�泥��ʴ�Ϊ��NaCl+NH4HCO3�TNaHCO3+NH4Cl
��4���Ȼ�狀�����淋��������������Ӻ���������ӵ��������������Ӻ������ӷ�Ӧ���ɰ�ɫ��������AgNO3����װ��ͼ��֪���տ�ʼ�ͱ���ͨ�������Ķ�����̼�����̼�����Ʒֽ��ֲ���������̼�����Կ��ö�����̼ѭ�����ã��ʴ�Ϊ��AgNO3��CO2
��������ѧ��Դ������������ַ�����������������ҵ�dz��л�ѧ��Ҫ��Ӧ��֮һ���ǿ����ص㣬���漰��ѧ����ʽ����д��
��2���ӷ�Ӧ��ĽӴ�����Ϸ������Ӳ������������жϣ�
��3�����������غ㶨��д����ʽ��
��4���������Ӻ������ӷ�Ӧ���ɳ����жϣ���Ӧ����������Ϸ�����
����⣺��1��̼�������ɽ������Ӻ�������ӹ��ɵģ����Σ��ʴ�Ϊ��C
��2��ʳ��ĥϸ��Ŀ�ľ�������Ӵ������ʹʳ����NH4HCO3��ַ�Ӧ���ѹ����Һ�����IJ����ǹ��ˣ��ʴ�Ϊ������Ӵ������ʹʳ����NH4HCO3��ַ�Ӧ������
��3���Ȼ��ƺ�̼����立�Ӧ����̼�����ƺ��Ȼ�泥��ʴ�Ϊ��NaCl+NH4HCO3�TNaHCO3+NH4Cl
��4���Ȼ�狀�����淋��������������Ӻ���������ӵ��������������Ӻ������ӷ�Ӧ���ɰ�ɫ��������AgNO3����װ��ͼ��֪���տ�ʼ�ͱ���ͨ�������Ķ�����̼�����̼�����Ʒֽ��ֲ���������̼�����Կ��ö�����̼ѭ�����ã��ʴ�Ϊ��AgNO3��CO2
��������ѧ��Դ������������ַ�����������������ҵ�dz��л�ѧ��Ҫ��Ӧ��֮һ���ǿ����ص㣬���漰��ѧ����ʽ����д��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�ٿ�ѧ�ҵ��㼣
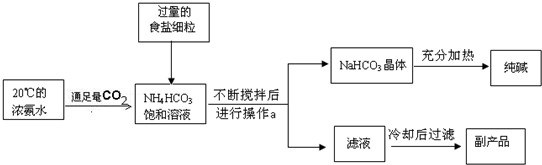
����1������IJ����Ǻ���һ�����һ�ѧ��ҵ��չˮƽ����Ҫָ�꣮������ʵ������������Ƽ����ԭ������Ҫ���̣�
��1��20��ʱ����Ũ��ˮ��ͨ�������Ķ�����̼�õ�NH4HCO3������Һ��
����NH4HCO3������Һ�м���ʳ��ϸ���������Ͻ��裬ֱ��NaHCO3�ᾧ������ϣ�
�۽������ľ�����ˣ��õ��������Һ��ϴ�Ӿ��壬Ȼ��������Թ��г�ּ��ȣ��õ�Na2CO3��
����۵���Һ�м���ʳ��ϸ��������NH4Cl���壬���ˣ��õ�NH4Cl��
����2���������ڲ�ͬ�¶��µ��ܽ�ȱ���
�Իش��������⣺
��1����ʵ�����У���ѡ����Ʊ�C02��Ӧ�Ļ�ѧ����ʽΪ ���ڹ�ҵ�����У�Ҫ������������ԭ��CO2����Ϊ���ʵ�õķ������� Ϊԭ�ϣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2��������Ϊ�����Ƽ�������ŵ㣬����Ϊ������ȷ���� ��
A�����������в��ַ�Ӧ�������Ϊԭ��ѭ��ʹ��
B������Ʒ��һ�ֵ���
C����Ӧ������Ҫ���ȣ��ɽ�Լ��Դ
��3��������еİ�ɫ��ĩ���Ƿ����Ȼ��Ƶķ����� ��
��4���ڢ��У�����ʳ�ε����룬��Һ�����NH4HCO3��NaCl�Ļ����Һ����������֮ǰ�������Һ�д��ڵ������У������ӷ��ű�ʾ����ͬ���� ���ڢ��У��������ľ�����˺���Һ���������ٵ������� ����Һ����Ȼ���ڵ������� �����з�Ӧ�Ļ�ѧ����ʽΪ ��
��5����20��ʱ����NH4HC03 21g�ı�����Һ�м�������ʳ�裮��ͨ������˵��������ʳ�ε����룬Ϊʲô����NaHC03������������û��NH4CI�������������������������ٿ�NaHCO3����������ʾ����С��û�м�����̲��÷֣������õ�����Է���������NH4HC03��79�� NaCl��58.5�� NaHC03��84��NH4CI��53.5����
����1������IJ����Ǻ���һ�����һ�ѧ��ҵ��չˮƽ����Ҫָ�꣮������ʵ������������Ƽ����ԭ������Ҫ���̣�
��1��20��ʱ����Ũ��ˮ��ͨ�������Ķ�����̼�õ�NH4HCO3������Һ��
����NH4HCO3������Һ�м���ʳ��ϸ���������Ͻ��裬ֱ��NaHCO3�ᾧ������ϣ�
�۽������ľ�����ˣ��õ��������Һ��ϴ�Ӿ��壬Ȼ��������Թ��г�ּ��ȣ��õ�Na2CO3��
����۵���Һ�м���ʳ��ϸ��������NH4Cl���壬���ˣ��õ�NH4Cl��
����2���������ڲ�ͬ�¶��µ��ܽ�ȱ���
| �¶� �� �ܽ�� |
10�� | 20�� | 30�� | 40�� | 50�� |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | ------ | ------ |
| NaHCO3 | 8.1 | 9.6 | 11.1 | 12.7 | ---- |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
��1����ʵ�����У���ѡ����Ʊ�C02��Ӧ�Ļ�ѧ����ʽΪ
��2��������Ϊ�����Ƽ�������ŵ㣬����Ϊ������ȷ����
A�����������в��ַ�Ӧ�������Ϊԭ��ѭ��ʹ��
B������Ʒ��һ�ֵ���
C����Ӧ������Ҫ���ȣ��ɽ�Լ��Դ
��3��������еİ�ɫ��ĩ���Ƿ����Ȼ��Ƶķ�����
��4���ڢ��У�����ʳ�ε����룬��Һ�����NH4HCO3��NaCl�Ļ����Һ����������֮ǰ�������Һ�д��ڵ������У������ӷ��ű�ʾ����ͬ����
��5����20��ʱ����NH4HC03 21g�ı�����Һ�м�������ʳ�裮��ͨ������˵��������ʳ�ε����룬Ϊʲô����NaHC03������������û��NH4CI�������������������������ٿ�NaHCO3����������ʾ����С��û�м�����̲��÷֣������õ�����Է���������NH4HC03��79�� NaCl��58.5�� NaHC03��84��NH4CI��53.5����
�ٿ�ѧ�ҵ��㼣
����1������IJ����Ǻ���һ�����һ�ѧ��ҵ��չˮƽ����Ҫָ�꣮������ʵ������������Ƽ����ԭ������Ҫ���̣�
��1��20��ʱ����Ũ��ˮ��ͨ�������Ķ�����̼�õ�NH4HCO3������Һ��
����NH4HCO3������Һ�м���ʳ��ϸ���������Ͻ��裬ֱ��NaHCO3�ᾧ������ϣ�
�۽������ľ�����ˣ��õ��������Һ��ϴ�Ӿ��壬Ȼ��������Թ��г�ּ��ȣ��õ�Na2CO3��
����۵���Һ�м���ʳ��ϸ��������NH4CI���壬���ˣ��õ�NH4CI��
����2���������ڲ�ͬ�¶��µ��ܽ�ȱ���
�Իش��������⣺
��1����ʵ�����У���ѡ����Ʊ�C02��Ӧ�Ļ�ѧ����ʽΪ ���ڹ�ҵ�����У�Ҫ������������ԭ��CO2����Ϊ���ʵ�õķ������� Ϊԭ�ϣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2��������Ϊ�����Ƽ�������ŵ㣬����Ϊ������ȷ���� ��
A�����������в��ַ�Ӧ�������Ϊԭ��ѭ��ʹ��
B������Ʒ��һ�ֵ���
C����Ӧ������Ҫ���ȣ��ɽ�Լ��Դ
��3��������еİ�ɫ��ĩ���Ƿ����Ȼ��Ƶķ����� ��
��4���ڢ��У�����ʳ�ε����룬��Һ�����NH4HCO3��NaCl�Ļ����Һ����������֮ǰ�������Һ�д��ڵ������У������ӷ��ű�ʾ����ͬ���� ���ڢ��У��������ľ�����˺���Һ���������ٵ������� ����Һ����Ȼ���ڵ������� �����з�Ӧ�Ļ�ѧ����ʽΪ ��
��5����20��ʱ����NH4HC0321g�ı�����Һ�м�������ʳ�裮��ͨ������˵��������ʳ�ε����룬Ϊʲô����NaHC03������������û��NH4Cl��
����1������IJ����Ǻ���һ�����һ�ѧ��ҵ��չˮƽ����Ҫָ�꣮������ʵ������������Ƽ����ԭ������Ҫ���̣�
��1��20��ʱ����Ũ��ˮ��ͨ�������Ķ�����̼�õ�NH4HCO3������Һ��
����NH4HCO3������Һ�м���ʳ��ϸ���������Ͻ��裬ֱ��NaHCO3�ᾧ������ϣ�
�۽������ľ�����ˣ��õ��������Һ��ϴ�Ӿ��壬Ȼ��������Թ��г�ּ��ȣ��õ�Na2CO3��
����۵���Һ�м���ʳ��ϸ��������NH4CI���壬���ˣ��õ�NH4CI��
����2���������ڲ�ͬ�¶��µ��ܽ�ȱ���
| �¶� �� �ܽ�� |
10�� | 20�� | 30�� | 40�� | 50�� |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | ------ | ------ |
| NaHCO3 | 8.1 | 9.6 | 11.1 | 12.7 | ---- |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
��1����ʵ�����У���ѡ����Ʊ�C02��Ӧ�Ļ�ѧ����ʽΪ
��2��������Ϊ�����Ƽ�������ŵ㣬����Ϊ������ȷ����
A�����������в��ַ�Ӧ�������Ϊԭ��ѭ��ʹ��
B������Ʒ��һ�ֵ���
C����Ӧ������Ҫ���ȣ��ɽ�Լ��Դ
��3��������еİ�ɫ��ĩ���Ƿ����Ȼ��Ƶķ�����
��4���ڢ��У�����ʳ�ε����룬��Һ�����NH4HCO3��NaCl�Ļ����Һ����������֮ǰ�������Һ�д��ڵ������У������ӷ��ű�ʾ����ͬ����
��5����20��ʱ����NH4HC0321g�ı�����Һ�м�������ʳ�裮��ͨ������˵��������ʳ�ε����룬Ϊʲô����NaHC03������������û��NH4Cl��