��Ŀ����
����Ŀ����һ�ձ���ʢ��22.3gNa2CO3��NaCl��ɵĹ��������100gˮ�ܽ��Ƴ���Һ������������μ���������������Ϊ20%��ϡ���ᣬ�������������������ϡ����Ĺ�ϵ����ͼ��ʾ����ش��������⣺
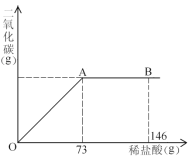
��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��������ǣ�д��ѧʽ�� ��
��2�����μ�ϡ���������ٲ�������ʱ���������������Ϊ���٣�
��3�����μ���73gϡ����ʱ����A��ʱ�����ձ��е���ҺΪ��������Һ���Լ����ʱ��Һ�����ʵ�����������
���𰸡���1��NaCl, HCl,
��2��8.8g
��3��13.1%
��������
�����������1��A��ʱ��ǡ�÷�Ӧ��B��ʱϡ������������ձ��е�������NaCl, HCl,
��2������3�������ɶ�����̼������Ϊx �������Ȼ��Ƶ�����Ϊy���������̼���Ƶ�����Ϊw
Na2CO3+2HCl==2NaCl+CO2��+H2O
106 73 117 44
w 73g��20% y x
73 : 44=73g��20% : x ���x=8.8g
73 �� 117=73g��20% �� y ���y=23.4 g
106 �� 73= w ��73g��20% ���w=21.2 g
��Ӧ��������Һ�����ʵ�����Ϊ����22.3g-21.2 g��+23.4 g=24.5 g
��Һ�����ʵ���������Ϊ��24.5 g����22.3g+100 g+73 g-8.8 g��=13.1%

����Ŀ����ͼA��F��ʵ�����Ʊ�ijЩ���������װ��ʾ��ͼ��

��1��װ��ͼ������a������Ϊ ��
��2��������غͶ������̻�Ϲ�����ȡ������д����Ӧ�Ļ�ѧ����ʽ��
��
��3��ʵ��������Bװ����ȡ������̼�Ļ�ѧ����ʽΪ ���������Gװ���ռ�������̼������Ӧ�� ��ͨ�����b����c��������Cװ�ô���Bװ����Ϊ����װ�ã�����Ҫ�ŵ��� ��
��4����ˮ���ռ����ſ������ռ�CO2�ıȽ����ֱ��������ͬ��2������ƿ�ռ���
�Ƚ���Ŀ | ��ˮ�� | �����ſ����� |
�ռ����������Է��� | ��ΪCO2�������ɺʹ�ˮ���ݳ�������Զ�������ܽ����ˮ��Ӧ�����ʣ����Կ�����ˮ���ռ��� | ��Ϊ_________��_________�����Կ��������ſ������ռ��� |
�ռ����̷��� | ����ˮ���ռ�CO2�����������ǣ� �� | ����������������������ɫ���ʼ���������ȷ����������ȼ��ľ���ƽ������ڻ���Ϩ��Ҳ����֤��������ȫ�ž� |
���ռ���CO2�ļ���ƿ�ڵ���������������ʯ��ˮ������ | �Ȼ��Ǻ���������ʱ��϶� | �Ȼ��Ǻ���������ʱ��ϳ� |
������ʵ��ɵý��� | ���ſ�������ȣ���ˮ�����ŵ��ǣ� �� | |
��5���������Ͽ�֪���ڽ������˳���λ�������Ľ���Cu���ڳ�������Ȼ������ϡ���ᡢϡ���ᷴӦ������������ɫ������Һ��Ӧ���仯ѧ����ʽΪCu + 4HNO3��Ũ����Cu��NO3��2 + 2NO2��+2H2O��
��Ӧ���ɵ�NO2�Ǻ���ɫ���д̼�����ζ���ж����塣������ͼ��ʾװ�ý���ʵ�飬��ش�
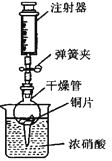
�ټ��װ�õ������ԣ��رյ��ɼУ�������ܷ������ˮ���ձ��У����۲쵽_______________________���������װ�õ����������á�
�ڴ��ɼУ���ע����������ȡ������ڵ����壬Ũ�������Ÿ��������������ֱ��������ͭƬ�Ӵ���ֹͣ����ע�������رյ��ɼУ��۲������ڵ������ǣ�_____________________________________��
������ʵ����ɺ���������NaOH��Һ���������գ���Ŀ����_____________________��

