��Ŀ����
ԭú�������в���ȫȼ�ջ�����CO��CO2�Ļ�����塣ͬѧ����ʵ����������ͼ��ʾ��װ�ü������ɵ����壬��������ͼ��ʾ��װ�ã�����������Ʒ�ԣ���

װ��A��������___��___��
װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ___��___��װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ___��___��
��3������װ����Ϻ������Բ���֮��������Ϊ����֮����___��___��
��4��Ϊ�ﵽʵ��Ŀ�ģ�������װ����Ϻ����ʵ�飬��ӿ�˳��Ϊ����������___��___�� ���a��h���ӿ���ţ�װ�ÿ��ظ�ʹ�ã�

װ��A��������___��___��
װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ___��___��װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ___��___��
��3������װ����Ϻ������Բ���֮��������Ϊ����֮����___��___��
��4��Ϊ�ﵽʵ��Ŀ�ģ�������װ����Ϻ����ʵ�飬��ӿ�˳��Ϊ����������___��___�� ���a��h���ӿ���ţ�װ�ÿ��ظ�ʹ�ã�
��1�� ��ˮ���� ��
��2�� Ca(OH)2 + CO2 ="===" CaCO3��+ H2O �� Fe2O3+3CO 2Fe+3CO2 ��
2Fe+3CO2 ��
��3��__û�н���β������___��
��4�� ef��cd��ef��ba��gh��ef ��
��2�� Ca(OH)2 + CO2 ="===" CaCO3��+ H2O �� Fe2O3+3CO
 2Fe+3CO2 ��
2Fe+3CO2 ����3��__û�н���β������___��
��4�� ef��cd��ef��ba��gh��ef ��
��������1����Ũ����������Կ��ǣ�
��2��������̼��C�еij���ʯ��ˮ��Ӧ����������е�CO�ܻ�ԭ��������
��3����һ����̼�ж����ǣ�
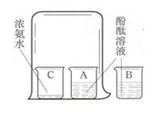
��4�����ݸ��Ե��������ӣ�A��������ˮ�֣�B�������ն�����̼��C���ڶ�����̼�ļ��飬D����һ����̼�ļ��飮
��𣺽⣺��1��A�е�Ũ�����������ˮ�֣�
�ʴ�Ϊ����ˮ������
��2��C�е�ʯ��ˮ�����ڼ��������̼�Ĵ��ڣ���ѧ��Ӧʽ�ǣ�Ca��OH��2+CO2�TCaCO3��+H2O��D����һ����̼�ļ��飬��ѧ��Ӧʽ�ǣ�Fe2O3+3CO 2Fe+3CO2��
2Fe+3CO2��
�ʴ�Ϊ��Ca��OH��2+CO2�TCaCO3��+H2O��Fe2O3+3CO 2Fe+3CO2��
2Fe+3CO2��
��3����CO�ж����ŷź����Ⱦ��������װ����û�д�����
�ʴ�Ϊ��û�н���β��������
��4�������������ef���������̼�Ĵ��ڣ���ͨ��cdʹ������̼��NaOH��Һ��ȫ���գ��ٴ�ͨ��ef���Ƿ��ж�����̼����ͨ��ba���տ��ܴ��ڵ�ˮ�֣��ٽ�ʣ���COͨ��gh��������ٴ���ef�����Ƿ������̼���ɣ�
�ʴ�Ϊ��ef��cd��ef��ba��gh��ef��
��2��������̼��C�еij���ʯ��ˮ��Ӧ����������е�CO�ܻ�ԭ��������
��3����һ����̼�ж����ǣ�
��4�����ݸ��Ե��������ӣ�A��������ˮ�֣�B�������ն�����̼��C���ڶ�����̼�ļ��飬D����һ����̼�ļ��飮
��𣺽⣺��1��A�е�Ũ�����������ˮ�֣�
�ʴ�Ϊ����ˮ������
��2��C�е�ʯ��ˮ�����ڼ��������̼�Ĵ��ڣ���ѧ��Ӧʽ�ǣ�Ca��OH��2+CO2�TCaCO3��+H2O��D����һ����̼�ļ��飬��ѧ��Ӧʽ�ǣ�Fe2O3+3CO
 2Fe+3CO2��
2Fe+3CO2���ʴ�Ϊ��Ca��OH��2+CO2�TCaCO3��+H2O��Fe2O3+3CO
 2Fe+3CO2��
2Fe+3CO2����3����CO�ж����ŷź����Ⱦ��������װ����û�д�����
�ʴ�Ϊ��û�н���β��������
��4�������������ef���������̼�Ĵ��ڣ���ͨ��cdʹ������̼��NaOH��Һ��ȫ���գ��ٴ�ͨ��ef���Ƿ��ж�����̼����ͨ��ba���տ��ܴ��ڵ�ˮ�֣��ٽ�ʣ���COͨ��gh��������ٴ���ef�����Ƿ������̼���ɣ�
�ʴ�Ϊ��ef��cd��ef��ba��gh��ef��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ