��Ŀ����
����Ŀ���±���ǰ18��Ԫ�������ڱ��еIJ�����Ϣ���ش��������⣺
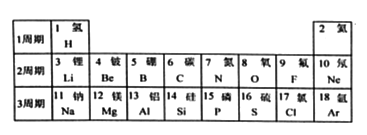
��1���ؿ��к�������Ԫ����______����Ԫ�ط��ţ�����Ԫ������__________Ԫ�أ���������ǽ���������
��2����д����16��Ԫ�ص�ԭ�ӽṹʾ��ͼ__________��ǰ18��Ԫ�����γɻ�������������Ԫ����____________����Ԫ�ط��ţ���
��3��13��Ԫ�ص���������H2SO4��Ӧ�Ļ�ѧ����ʽΪ_______________��
6��Ԫ�ص����������������NaOH��Ӧ�Ļ�ѧ����ʽΪ_______________��
��4������1�����⣬ͬ����Ԫ�ص�ԭ�ӽṹ����ͬ����__________________��
���𰸡� O �ǽ��� ![]() C Al2O3+3H2SO4= Al2(SO4)3+3H2O CO2+2NaOH= Na2CO3+H2O ���Ӳ�����ͬ
C Al2O3+3H2SO4= Al2(SO4)3+3H2O CO2+2NaOH= Na2CO3+H2O ���Ӳ�����ͬ
����������1���ؿ��к�������Ԫ������Ԫ�أ���Ԫ�����ڷǽ�����
��2������ԭ�ӽṹʾ��ͼ�Ļ���������ԭ�ӵĽṹʾ��ͼ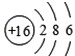 ���γɻ�������������Ԫ����̼��
���γɻ�������������Ԫ����̼��
��3��13��Ԫ��Ϊ��Ԫ�أ�����������Ϊ�������������ᷴӦ�����κ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Al2O3+3H2SO4=Al2��SO4��3+3H2O��6��Ԫ����̼�����������ΪCO2��������NaOH��Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O��
��4�������ͼ����֪��ͬһ����Ԫ��ԭ�ӽṹ�Ĺ�����ԭ�ӵ�������������1���ε�����8�����Ӳ�����ͬ��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�����Ŀ��������ʵ��Ӧ�Ľ����������
��� | ��ʵ | ���� |
A | �ڻ��п��ŵ����� | �����ڲ����˶� |
B | �����۲���ֱ�ӹ۲쵽CO2���� | CO2���Ӻ�С |
C | 50����ˮ��50�����ƾ���ϣ���Ϻ������С��100���� | ����֮���м�� |
D | �����ȱ�Ϊˮ��ˮ���ȱ�Ϊˮ���� | ���ӿ����ٷ� |
����Ŀ����ȥ���������л��е��������ʣ�������Ϊ���ʣ������÷�������ȷ����
���� | ���ӷ��� | |
A | N2�� O2 �� | ������ͨ�����ȵ�ͭ�� |
B | CaCO3��NaCl�� | �ܽ⣬���� |
C | CO��CO2�� | ������ͨ������������Һ |
D | Na2CO3��Һ��NaOH�� | �μ�ϡ������ǡ����ȫ��Ӧ |
A. A B. B C. C D. D