��Ŀ����
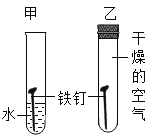
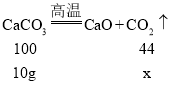
����Ŀ��ijͬѧ��ȡ 10g ̼��Ʒ�ĩ����ͼ��ʾ����ʵ�顣

��1������ 10g ̼�����ȫ�ֽ����ɶ�����̼�����ʵ����������ݻ�ѧ����ʽ��ʽ���㣩_____
��2������һ��ʱ�����ֹͣʵ�飬Ӧ��_____��
��3���Լ��Ⱥ��ʣ����� A ����ʵ�飬��������ͼ��ʾ����Һ B ��������_____������III ����������Ļ�ѧ����ʽ��_____��ʣ����� A �ijɷ���_____��
��4����ʹ�÷�̪��Һ��ϡ����Ҳ����ȷ��ʣ�����A �ijɷ֣�д�����������ۼ�����_____��
���𰸡�0.1mol �����ܴ�ʯ��ˮ��ȡ�� Ca(OH)2 CaCO3+2HCl=CaCl2+H2O+CO2�� CaO��CaCO3 ����ʣ�����A������m�����������غ㶨�ɣ���mΪ10g��ʣ�����A�ɷ�ΪCaCO3����5.6g<m<10g����ΪCaO��CaCO3����mΪ5.6gʣ������������ƣ��������ɣ�
��������
̼��Ƹ������������ƺͶ�����̼�������ƺ�ˮ��Ӧ�����������ƣ�̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��
��1����10g ̼�����ȫ�ֽ����ɶ�����̼������Ϊx
![]()
![]()
��10g ̼�����ȫ�ֽ����ɶ�����̼�����ʵ���Ϊ![]()
��2������һ��ʱ�����ֹͣʵ�飬Ϊ��ֹˮ������Ӧ�Ƚ����ܴ�ʯ��ˮ��ȡ����
��3���Լ��Ⱥ��ʣ����� A ����ʵ�飬�����ƺ�ˮ��Ӧ�����������ƣ���ҺB�μ���ɫ��̪��Һ��죬˵����Һ�Լ��ԣ�����ҺB���������������ƣ�Ca(OH)2��������III ����������ķ�Ӧ��̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2������ҺB���������������ƣ�ʣ�����A����ˮ��������ҺB�Ͱ�ɫ����C��̼��Ʋ�����ˮ��˵��A�к���̼��ƺ������ƣ���ʣ����� A �ijɷ��������ƺ�̼��ƣ�CaO��CaCO3����
��4����ʹ�÷�̪��Һ��ϡ����Ҳ����ȷ��ʣ�����A �ijɷֵĹ��̣�����ʣ�����A������m�����������غ㶨�ɣ���mΪ10g��ʣ�����A�ɷ�ΪCaCO3����5.6g<m<10g����ΪCaO��CaCO3����mΪ5.6gʣ������������ƣ��������ɣ���

����Ŀ���Ա�ʵ��Ϳ��Ʊ������ǻ�ѧ�г��õ�̽������ͷ����������Ŀ�ѧ������
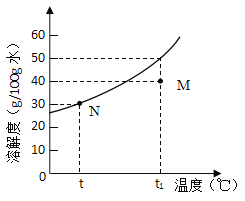
��1��20��ʱ��ijʵ��С��ȡ��ͬ����������أ��ֱ���뵽100gˮ�У�����ܽ��������Һ���������������ص�������Ӧ��ϵ���:
ʵ��һ | ʵ��� | ʵ���� | ʵ���� | |
����Ѻ�ص�����/g | 20.0 | 25.0 | 30.0 | 35.0 |
������Һ������/g | 120.0 | 125.0 | 130.0 | 131.6 |
�ٸ����ϱ����ݣ�ʵ������õ���ҺΪ____________������������Һ��������������Һ������
������Ҫ�۲쵽ʵ���ĵ�35g�����ȫ���ܽ���ˮ�У��ɲ�ȡ�ķ�����__________��
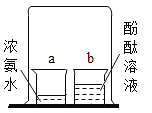
��2����ͼ������Ӻ��������˶���ʾ��ͼ��
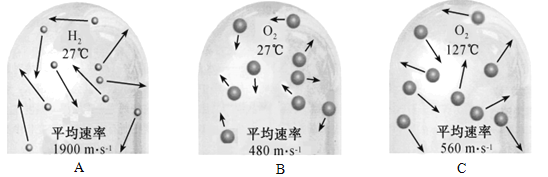
����A��B��C�У��ܱȽϵó����¶�Խ�ߣ������˶�����Խ��������__________������ĸ��ţ���
�ڴ�ͼ�пɼ���Ӱ������˶����ʵ����س��¶��⣬����__________�йء�
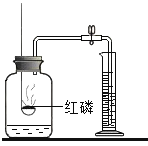
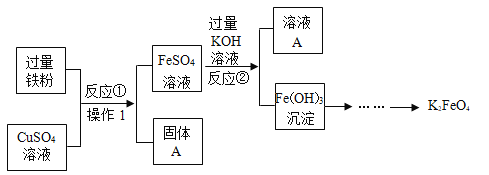
����Ŀ����������ʵ������ȡ����ķ������ռ�װ�ã�ѡ��I����II���������𣬶��߾����𣬰���I�����мƷ֡�
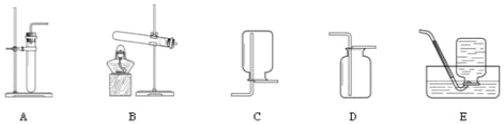
I | II |
��1��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽ��_____������װ��ѡ��_____�� ��2��������ѡ��E�����ռ�����ԭ����_____�� | ��1��ʵ������ȡ������̼�Ļ�ѧ����ʽ��_____���ռ�װ��ѡ��_____�� ��2��������̼������������_____�� |
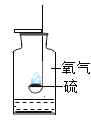
����Ŀ��������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ���õ��ǣ�������
A | B | C | D | |
ʵ��װ�� |
����������ȼ�� |
�ⶨ�������������� |
��˿��������ȼ�� |
��ˮ���ռ����� |
���� | ����ƿ�е�ˮ�����շų����� | �����е�ˮ��ͨ��ˮ����ı仯�ó�O2��� | ����ƿ�е�ˮ����ȴ�����������ֹ����ƿը�� | ����ƿ�е�ˮ���Ƚ�����ƿ�ڵĿ����ž�������ڹ۲�H2��ʱ�ռ��� |
A.AB.BC.CD.D